
DISCO is a VUV to visible beamline dedicated to biochemistry, chemistry and cell biology. The spectral region is optimised between 60 and 700 nm with conservation of the natural polarization of the light.
DISCO offers three experimental endstations to the scientific communities: SRCD (circular dichroism using synchrotron radiation), APEX (ionisation experiment at atmospheric pressure) and DUV imaging.
Our last publications on twitter
Team






Photo Disco team et Associés
Disco team
Associés
Marie-Françoise Devaux : IR INRA - chimiométricienne
Technical data
Flat and toroid mirrors with cold finger for M0. Beam divided in three for the endstation
Dipole Radiation: 40 mrd H x 10 mrd V
|
BRANCHES
|
SRCD
|
POLYPHEME
|
|
|
Energy domains
|
120-400 nm
|
180-1000 nm
|
|
|
Resolution in energy
|
0.1 nm
|
0.5 nm
|
|
|
Flux @ first optical element
|
|
10 e+14 Phot/s/0.1%bw @ 100 nm
|
|
|
Samples environnement
|
Cellules en CaF2
|
Inverted microscope |
|
|
Beamsize @ sample
|
4x4 mm2
|
0.1x0.1 μm2
|
|
|
Detectors
|
PMTs
|
PMTs, time gated CCD
|
Circular
DISCO beam is extracted from a dipole : D04.1 (1.71 T). A 40 per 10 mrad opening is taken.
The imaging endstation comprises two inverted microscope with pre and post monochromators and three detectors : one PMT, one phase gated CCD camera and one APD.
The mass spectrometry endstation comprises one commercial mass spectrometer with an injection port and an analysis station.
The CD endstation comprises one optical bench with a photoelastic modulator, a sample holder and a PMT.
A tiny lab dedicated and very close to DISCO includes a cell laboratory and a mini biochemistry lab.
The huge biochemistry lab for users is located closely undercover from the beamline.
Scientific Opportunities
|
Material sciences |
Microscopic Analysis defaults inside optical materials ; prebiotics and fossils inclusions topology inside geological materials. |
|---|---|
| Biochemistry | Proteins and DNA structures and dynamics ; sugar structures ; Mass spectrometry of proteins |
| Biology , Biomedics |
Imaging biochemical reactions inside individual cells with fluorescent proteins; Autofluorescence of tumoral cells and tissues; Study of drug distribution inside human tissues without additional labelling ; diagnosis of hydrophobic molecules |
DISCO is a VUV to visible beamline of phase 2. First users were received in september 2009.
Three endstations are operated around a common scientific topic : Biomolecular investigations, with special emphasis on proteins, particularly membrane proteins:
- A circular dichroism endstation in the VUV range which opens new fields in the investigation of biological molecules, with, in particular, the possibility to follow rapid kinetics such as proteins folding and unfolding in real time,.
- An endstation for mass spectrometry of non-soluble molecules based on VUV photoionization with new opportunities for proteomics and photochemistry of hydrophobic molcules.
- An imaging endstation for biological (living cells) and material applications with new possibilities of excitation and detection, also allowing biomedical investigation of normal and tumoral tissues moreover, new paradigms in autofluorescence diagnosis appear and "abandonned" molecules that fluoresce only in the UV can be used as probes.
Two endstations are always working in parallel, the imaging one and SRCD or APEX.
See also the scientifical section in SOLEIL: HelioBio
 1. SRCD allows to record circular dichroism spectra in solution down to 165 nm even with very aborbing buffers and films down to 120 nm. Very fast acquisition of datas about folding and stability of biomacromolecules.
1. SRCD allows to record circular dichroism spectra in solution down to 165 nm even with very aborbing buffers and films down to 120 nm. Very fast acquisition of datas about folding and stability of biomacromolecules.
2. IMAGING : two microscopes (wiki)
- a microspectrofluorimeter (POLYPHEME) for recording fluorescence spectral images of molecules absorbing between 190 and 400 nm. allows femtoliter spectral recording.
- a full field, fully automatized inverted microscope (TELEMOS), for fast imaging of live cells or any other specimen that do present fluorescence in UV. Often used in Z-Stacks configurations.
- Numerous molecules present a specific fluorescence when excited in UV (190 to 400 nm), here are some examples that we did encounter: aromatic amino acids of course, but also NADH, DHE (that may be sustitued to cholesterol for studying membranes), ferulic acids, lignin, G quadruplexes, polyenes, metal oxydes, ...
3. APEX : to ionize at atmospheric pressure the biomolecules between 5 and 20 eV. This ionization can be coupled with a mass spectrometer.
SRCD
DISCO Beamline SRCD endstation provides users with a fully flexible circular dichroism spectrometer in the UV. Reference publication of the endstation :
M. Réfrégiers, F. Wien, H.-P. Ta, L. Premvardhan, S. Bac, F. Jamme, V. Rouam, B. Lagarde, F. Polack, J.-L. Giorgetta, J.-P. Ricaud, M. Bordessoule and A. Giuliani DISCO synchrotron-radiation circular-dichroism endstation at SOLEIL J. Synchrotron Rad. (2012). 19, 831-835
SRCD advantages
Synchrotron radiation extends toward higher energies the spectral domain of circular dichroism. Moreover the exterme stability of the beam and the wide accessible spectral range are real asset for difficult solvants.
Data analysis
We strongly recommand you to analyse your data with BestSel a novel method for the secondary structure determination and fold recognition from protein circular dichroism spectra. More about the algorithm at Micsonai et al., Proc. Natl. Acad. Sci. USA (2015) doi:10.1073/pnas.1500851112.
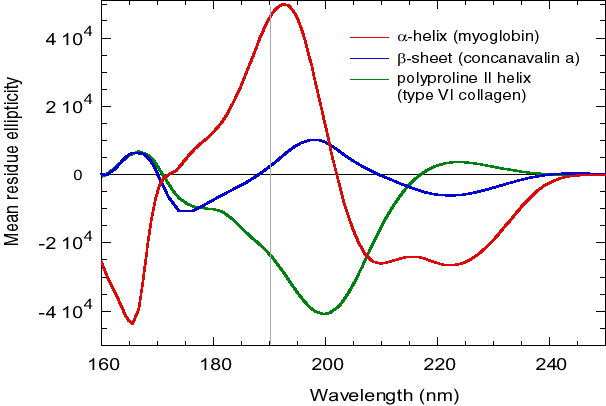
Figure 1 d'après Wallace, B.A. and Janes, R.W. (2001) SRCD Spectroscopy of Proteins: Secondary Structure, Fold Recognition and Structural Genomics. Curr. Opin. in Chemical Biology, 5: 567-571
Figure 1 d'après Wallace, B.A. and Janes, R.W. (2001) SRCD Spectroscopy of Proteins: Secondary Structure, Fold Recognition and Structural Genomics. Curr. Opin. in Chemical Biology, 5: 567-571
The high synchrotron brilliance allows to work with turbides and very absorbing samples and buffers.
Useful infos
Samples are sandwiched between CaF2 coverslips, a transparent material in the VUV. Optical pathways may vary from 2,5 μm to 50 μm.
- Typical concentrations are between 0,1 and 10 mg/ml.
- Only few microliters are necessary: from 0.5 μl to 15 μl for optical pathways comprised between 2.5 μm and 50 μm.
- Full thermal scans from 0 to 100°C.
Some buffers and salts absorb a lot in DUV and shall be replaced. This table adapted from Franz X. Schmid, p. 251 « Protein structure – a practical approach », IRL Press, Oxford 1989, gives absorbances of salts and buffers at 0.01 M in 0.1 cm pathlength.
| Substance | transparent jusqu'à |
abs 200 nm | abs 180 nm |
|---|---|---|---|
| NaClO4 | 170 nm | 0 | 0 |
| NaF, KF | 170 nm | 0 | 0 |
| Ac. Borique | 180 nm | 0 | 0 |
| NaCl | 205 nm | 0.02 | >0.5 |
| Na2HPO4 | 210 nm | 0.05 | >0.5 |
| NaH2PO4 | 195 nm | 0 | 0.15 |
| Na Ac. | 220 nm | 0.17 | >0.5 |
| Glycine | 220 nm | 0.1 | >0.5 |
| Diethyl Amine | 240 nm | >0.5 | >0.5 |
| NaOH | 230 nm | >2 | >2 |
| AcideBorique-NaOH | 200 nm | 0 | 0.3 |
| Tricine | 230 nm | 0.44 | >0.5 |
| Tris | 220 nm | 0.13 | >0.5 |
| Hepes | 230 nm | 0.5 | >0.5 |
| Pipes | 230 nm | 0.5 | >0.5 |
| Mops | 230 nm | 0.34 | >0.5 |
| Mes | 230 nm | 0.3 | >0.5 |
| Cacodylate | 210 nm | 0.2 | >0.5 |
Bientôt, ici, un logiciel avancé pour l'analyse des spectres de dichroïsme circulaire: CiDiPy
IMAGING

Waiting for a detailed manual (one day ?), you will find here, some useful infos on the two microscopes available at DISCO beamline.
Both microscopes receive monochromatic light quickly tuneable between 200 and 600 nm. The first, POLYPHEME, is dedicated to DUV fluorescence microspectroscopy fluorescence, the second, TELEMOS, is a DUV fast full field microscope with Z scanning for 3D reconstruction.
Référence: Jamme, F., Villette, S., Giuliani, A., Rouam, V., Wien, F., Lagarde, B., & Refregiers, M.Synchrotron UV Fluorescence Microscopy Uncovers New Probes in Cells and Tissues. Microscopy and Microanalysis, 2010, 16(5): 507-514
Samples preparation:
Both microscopes are inverted, POLYPHEME is a IX71, TELEMOS is an axioobserver Z1. Most samples will therefore be positioned upside downover a quartz coverslip. Our two main objective use immersion glycerin, it is also possible to observe samples with a air long working distance objective.
- Cells
Nous possédons une cellule attofluor (Invitrogen). Elle permet de recevoir des cellules qui ont poussé sur une lamelle ronde en suprasyl (nous contacter si vous désirez recevoir de telles lamelles). Si vous possédez vos propres chambres d'incubation, n'hésitez pas à nous contacter. - Tissues
Nous conseillons de déposer les biopsies ou coupes sur des lames sans monter de lamelles sauf si vous avez des lamelles UV. Les épaisseurs peuvent varier entre 1 et 10 µm environ, pour des demandes spécifiques n'hésitez pas à nous contacter. Certains milieux de montage transparents aux UVs peuvent être utilisés. - Fixation protocole (from Slavka Kascakova) :
- Before seeding the cells on quartz slides, the slides need to be sterile. It's sufficient to sterilize them with 70% of EtOH (let slides from 30 min - 1 hour in 70% of EtOH and then let them dry under the flow). You can eventually, once the slides are dried, let them under the UV light for 30 min.
- Afterthat the quartz slides are placed into the well plates or Petri dish and cells are deposit on it with its culture medium. The plate is then placed into the incubator with 5% CO2 and 37°C. We are making sure that cells nicely adapt on slide and proliferate.
- Individual cell treatment.....Incubation with nanoparticles, drug or whatever product for different periods. After incubation, cells are washed with PBS or medium without FCS.
- Cell treatment is followed by fixation for microscopic observation: Slide is gently removed from well and placed into the Petri dish with 4 % formalin in PBS for 20 min at room temperature. After this period, cells are then washed for 5 seconds with distilled water, to remove the residual PBS from the surface of the cells. The cells are then dried under ambient conditions and stored in fridge at 4°C. (For drying process, one can eventually deposit the slides on Whatman lens cleaning tissue (http://www.whatman.com/PRODLensCleaningTissue.aspx) (Whatman paper leaves no fibers and it's not damaging optical surfaces). It's also important to mention, that manipulation with slide should be very gentle - very easily one can do scratches on the surface of slide or break the slide.
Platine PI de POLYPHEME
PI 542.2CD – 80x80 mm aperture – 100x100 μm travel range
Liste des objectifs
| 40x Ultrafluar Zeiss Glyc N.A: 0.6 Working distance: 0.36 Features: Infinity corrected |
100x Ultrafluar Zeiss Glyc N.A: 1.25 Working distance: 0.17 Features: |
| 40x OFR LMU N.A: 0.5 Working distance: 1 mm Features: Infinity corrected |
10x Ultrafluar Zeiss N.A: 0.2 Working distance: 7.4 Features: Infinity corrected |
Liste des filtres accessibles sur TELEMOS :
- Dichroïques
DM300, DM400, DM505 - Bandpass and longpass filtres
Un panel de filtres passes bandes à l'émission sont utilisables. Ce sont des filtres 1" provenant de chez Omega Filters. Vous pouvez aussi amener vos propres filtres ou discuter avec l'équipe de ligne pour des achats spécifiques pouvant profiter au plus grand nombre.
| Nom | XF1000 | XF3000 | XF1076 | QMAX/EM420-480 | XF3075 | QMAX/EM510-560 | XF3085 |
|---|---|---|---|---|---|---|---|
| Description | OMEGA | OMEGA | OMEGA | OMEGA | OMEGA | OMEGA | OMEGA |
| Nom |
XF1009 |
QMAX/EM600-650 | QMAX/EX355-405 | QMAX/EX530-570 |
|---|---|---|---|---|
| Description | OMEGA | OMEGA | OMEGA | OMEGA |
Liste of excitables fluorochromes in DUV :
• Pour une liste détaillée de fluorochromes endogènes, nous vous conseillons de consulter :
Wagnieres G.A., Star W.M. and Wilson B.C. (1998) ln Vivo Fluorescence Spectroscopy and Imaging for Oncological Applications. Photochem. Photobiol. 68, 603-632
Les logiciels utilisés sur les deux microscopes :
- Pour l'acquisition de données
- En microspectrofluorimétrie : Labspec
- En imagerie plein champ et 3D : µManager - Pour l'analyse
Pour le traitement d'images spectrales :
- Labspec
- Matlab
Pour le traitement d'images 2D : ImageJ
Pour la déconvolution d'images et la reconstruction d'images 3D: Huygens
Recording mosaïcs
1- In micromanager, check that the pixel size is calibrated
| Objective | Pixel size |
|---|---|
| 10x zeiss ultrafluar | 1.2 um |
| 100x zeiss ultraflar | 0.153 um |
| 40x Zeiss Ultrafluar | 0.313 um |
2- perform slide explorer until you obtain the area of interest in transmission mode, save it
3- define a rectangle (ROI) with imageJ tools and click on ROI -> POS
4- launch a multidimensionnal acquisition with multiposition on but only one filter.
How to perform mosaïcs of images for large area mapping on TELEMOS microscope at DISCO Beamline:
Recording mosaïcs
1- In micromanager, check that the pixel size is calibrated (! for a binning of 1)
| Objective | Pixel size |
|---|---|
| 100x zeiss ultrafluar | 0.153 um |
| 40x zeiss ultraflar | 0.313 um |
| 10x Zeiss Ultrafluar | 1.2 um |
2- perform slide explorer until you obtain the area of interest in transmission mode, save it
3- define a rectangle (ROI) with imageJ tools and click on ROI -> POS
4- launch a multidimensional acquisition with multiposition on but only one filter.
Stitching mosaic
1- you will first need to reorder your data, this can easily be made by opening the saved folder in micromanager and clicking on save stack
2- in FIJI, use the plug-in:Stitchin:Deprecated:Stitch sequence of grid of images
3- use the following options : overlap 12% for the 10x.
4- for post-treatment of mosaics, we recommend the use of BaSiC
Soon here: ICY protocols for image analysis; because, as ICY's team, we believe in reproducible research.
That's it !
Do not forget to save the result.
APEX
La branche APEX de la ligne DISCO délivre des photons VUV entre 350 et 60 nm à pression atmosphérique sous une atmosphère de gaz rare (argon ou néon).
La branche peut être couplée à une source de photoionisation à pression atmosphérique (APPI pour atmospheric pressure photoionisation) pour des analyses par spectrométrie de masse. Le spectromètre de masse utilisé dans ce cas est un QStar pulsar i de la société ABSciex. La gamme de masse accessible va jusqu’à m/z 10000 avec un pouvoir de résolution de 9000 dans les deux modes d’ionisation.
Le pompage différentiel est décrit dans :
Giuliani, A., Yao, I., Lagarde, B., Rey, S., Duval, J. F. L., Rommeluere, P., Jamme, F., Rouam, V., Wien, F., de Oliveira, C., Ros, M., Lestrade, A., Desjardins, K., Giorgetta, J. L., Laprévote, O., Herbeaux, C., & Refregiers, M. A differential pumping system to deliver windowless VUV photons at atmospheric pressure. Journal of Synchrotron Radiation, 2011, 18, on-line first.
Les possibilités sont décrites dans :
Giuliani, A., Giorgetta, J. L., Ricaud, J. P., Jamme, F., Rouam, V., Wien, F., & Refregiers, M.Atmospheric pressure photoionization using tunable VUV synchrotron radiation. Nuclear Instruments and Methods B, 2012, on-line first.
Quelques applications possibles des photons VUV...
DISCO receives users since septembre 09 on three endstations.
Communitiess
Projects Submission Help
- Do not forget to contact one of the beamline scientists (see Team).
- Energy domains are function of the endstations
- Sample environments may be defined with your local contact.
- Beamtime request justification is crucial.
- How to cite the beamline in your articles?
Nous vous recommandons de
• Cite the beamline in your material and methods (DISCO beamline), with the following reference Giuliani et al, Journal of Synchrotron Radiation 2010;
• Cite the endstation on which you recorded your experiment with the corresponding reference;
• Thank SOLEIL in the acknowledgement, with your project numbers;
• Thank the local contact if you want.
Do not forget to upload your publications linked to SOLEIL in the sunset.
Websites & publications of interest
- Sur le CD
- Protocoles d'analyse
- Petit cours de CD - Sur la spectrométrie de masse
- Laprévote's Team
- Mass spectrometry lessons (in french) - Sur l'imagerie de fluorescence
- SYCLOPS
- Réseau d'Imagerie Cellulaire Paris XI
- Réseau Technologique de microscopie photonique du CNRS - Site INRA dédié aux expériences sur synchrotron
http://www.inra.fr/inra_cepia/vous_recherchez/des_plates_formes_et_des_outils/soleil
Users Publications
Link to users publications (reported by a U)
Our users do have talent !
- En mars 2010, Stéphane Pallu et Gaël Rochefort de l'Inserm (CHRO, Orléans) ont tourné un film sur leur travail à DISCO : L'Ostéoporose à la lumière de SOLEIL
- Béatrice Matot (CEA Saclay) a gagné le prix du poster 2010 au user meeting de SOLEIL.
- Francis Canon (INRA, Montpellier) a réalisé un superbe reportage photo sur la ligne lors de sa construction.
Lexicon
- DISCO: Dichroism, Imaging and mass Spectrometry for Chemical and biOlogical systems (Inspired author : L. Nahon)
- CD BIO: Old name of DISCO
- CD: Circular Dichroism
- SRCD: Synchrotron Radiation Circular Dichroism
- POLYPHEME:Cyclop was already taken... this is DISCO Microscope nickname, son of Poseidon he does'nt know nobody
- TELEMOS: Another Cyclop with the gift of telling the future, son of Protée
Related Topics
Complementary beamlines at SOLEIL
DISCO experiments is complementary to SMIS (Infrared microscopy) for cellular biology. SRCD and Mass spectrometry are complementary to SWING, PROXIMA I and PROXIMA II for structural biology and biochemistry.
Other SRCD beamlines in the world
| B23, Diamond | UV1, ASTRID | 3m NIM1, BESSY2 | BL15, HiSOR |
| Oxon ,UK | Aarhus, Dk | Berlin, DE | Hiroshima, JP |
| BESSY 2 new | NSRL | CD12 revamped | 4B8,IHEP |
| Berlin, DE | Hefei, CHINA | Anka | Beijing, CHINA |
The in-house researches of the DISCO Team concern the interactions of light with biomolecules. They are funded by ANR, Région Centre and PRES UniverSud.
Research Areas
 Les recherches développées par l'équipe concernent les interactions de la lumière avec les biomolécules. Elles sont soutenues par l'ANR, la Région Centre et le PRES Universud.
Les recherches développées par l'équipe concernent les interactions de la lumière avec les biomolécules. Elles sont soutenues par l'ANR, la Région Centre et le PRES Universud.
- Photochomie et photobiologie
- Protéines intrinsèquements peu structurées
- Suivi de médicaments non marqués
See also scientifical section in SOLEIL : HelioBio
Fundings
IMI : projet TRANSLOCATION
ANR : SRMS2, SOPOL, METABACT
ANS INRA :
Participation à l'axe NBS de C'Nano, à l'axe E du RTRA Triangle de la Physique.
 |
 |
 |
 |
Collaborations
- CBM (Orléans)
- BIA (Nantes)
- UMR MD1 (Marseille)
- UMR 8601 (Paris V)
- IPCM (Paris VI) - H. Dossmann





