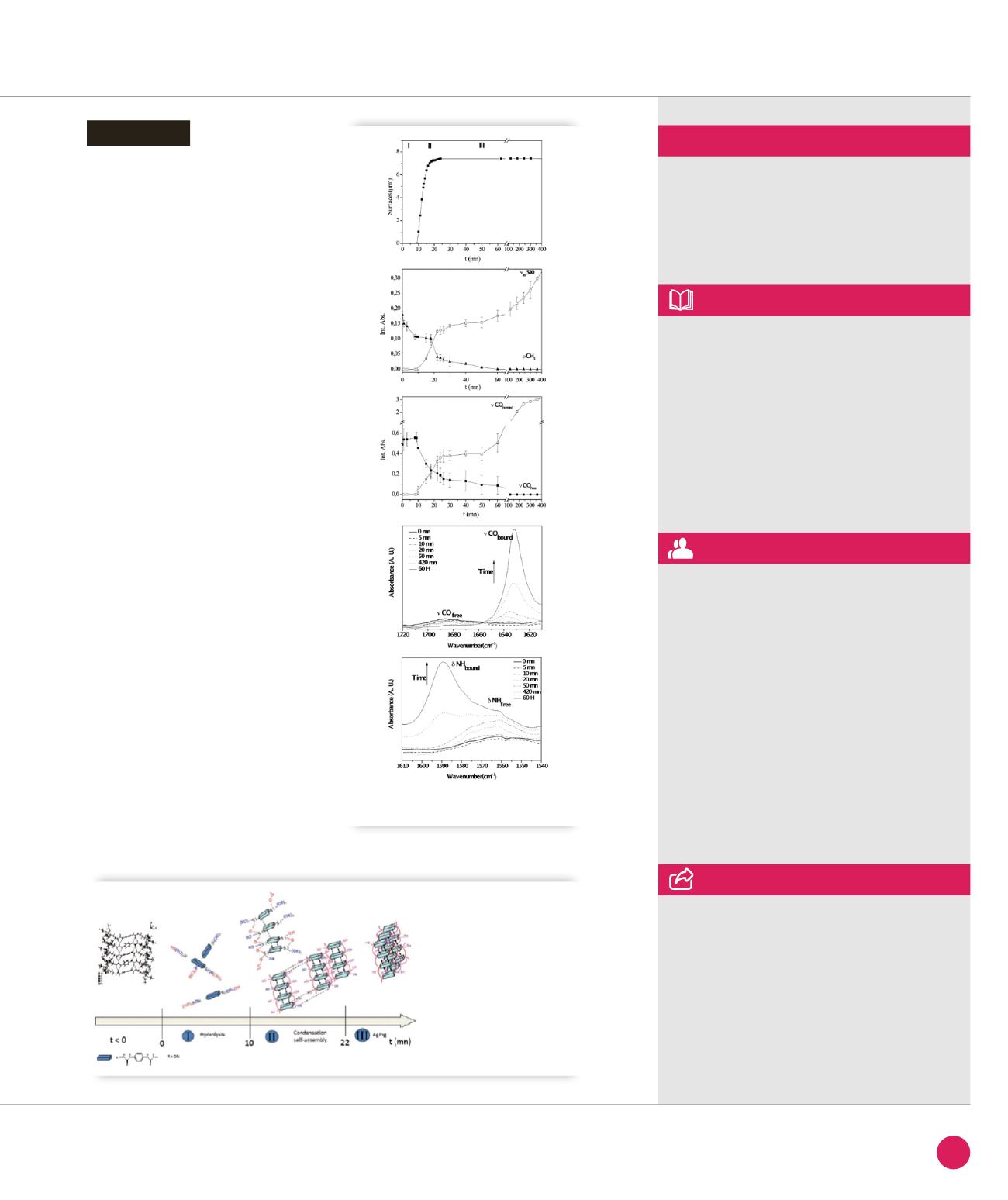
The techniques used (OM, IR and LS)
to investigate the formation of a self-
assembled
BS
during sol-gel processing
clearly demonstrate a competition
between covalent Si-O-Si bond and urea
H-bonding interactions producing
needle-like crystals.
A mechanism describing the formation
of the organized
BS
is proposed:
• Hydrolysis of
P
proceeds without
significant condensation until around
50 % of the alkoxy groups are
consumed;
• When the critical supersaturation
concentration of the hydrolysed
P
is reached, formation of the organized
BS
starts via a concerted process
involving intermolecular H-bonding
of urea groups and condensation
of adjacent silanol/alkoxy species
to form siloxane species. Subsequent
growth of the organized material
is mediated by direct “attachment”
of precursor molecules at the solid/
solution interface;
• During aging, local reorganization
occurs due to mechanical stress caused
by increasing polycondensation within
the needles leading to the crystals.
Figure
➌
shows a tentative representation
of the self-structuring of
BS
in the 3
domains.
Conclusion
➌
Proposed mechanism
for the self-assembled
structure of
BS
CRISTAL beamline
ASSOCIATED PUBLICATION
Self-assembly of bridged silsesquioxanes:
modulating structural evolution via cooperative
covalent and non-covalent interactions
G. Creff, B.P. Pichon, C. Blanc, D. Maurin,
J-L. Sauvajol, C. Carcel, J.J.E. Moreau,
P. Roy, J.R. Bartlett, M. Wong Chi Man*
and J-L.Bantignies
Langmuir 29 (2013), 5581
REFERENCES
[1] J.J.E. Moreau et al. Angew Chem. Int. Ed.,
43 (2004), 203.
[2] P. Dieudonné et al. Small. 2009, 5, 503.
[3] JL. Bantignies et al. J. Phys.Chem. B,
110 (2006), 15797
[4] G. Creff et al. PCCP 14 (2012), 5672
* Laboratoire Architectures Moléculaires
et Matériaux Nanostructurés - ICG Montpellier
(UMR 5253) CNRS- UMII-ENSCM- UMI,
ENS de Chimie de Montpellier, 8 rue de l’Ecole
Normale, 34296 Montpellier Cedex 5, France
CORRESPONDING AUTHOR
➋
Three domains obtained by OM, DLS and MFTIR
experiments
37
SYNCHROTRON
HIGHLIGHTS
2013


