Active sites of
metalloproteins in solution
revealed by vibrational
spectroscopy
New information on protein’s structure,
intra- and intermolecular hydrogen bonds,
or metal-ligand bond properties can be
unraveled in the Far IR and TeraHertz
domain (600 - 3 cm
-1
or 18 - 0.1 THz).
Using Cu-azurin placed in a short path-
length electrochemical cell adapted for
transmission spectroscopy at the beamline,
we show that the brilliance and stability
of the Far-IR beamline AILES [1,2] enables
to detail molecular properties of metal
sites or metal redox states of proteins.
Moreover it allows extracting from a complex
background hydrogen bonding signatures
directly relevant to the protein function.
Vibrational analysis of proteins
or biological molecules provides new
information on biomolecular structures,
dynamics and properties of intra- and
intermolecular hydrogen bonds. Bending
vibrational modes of amino acids as well
as metal-ligand vibrations also absorb in
the Far-IR, which is particularly appealing
for probing metal active sites
in metalloproteins [3,4].
Far-IR absorption spectra of proteins
in aqueous solution are dominated by
the strong absorption of water. Due
to the weakness of the signals to probe,
typically of the order of 10
-5
to 10
-3
unit
of absorbance, difference spectroscopy
is needed to identify modes associated
with active sites.
Only vibrations of chemical groups
selectively perturbed by the Cu redox
switch contribute in electrochemically-
induced difference spectra. Indeed,
in figure
➊
and
➋
, well-defined bands
are observed for the CuII state (negative)
and for the CuI state (positive).
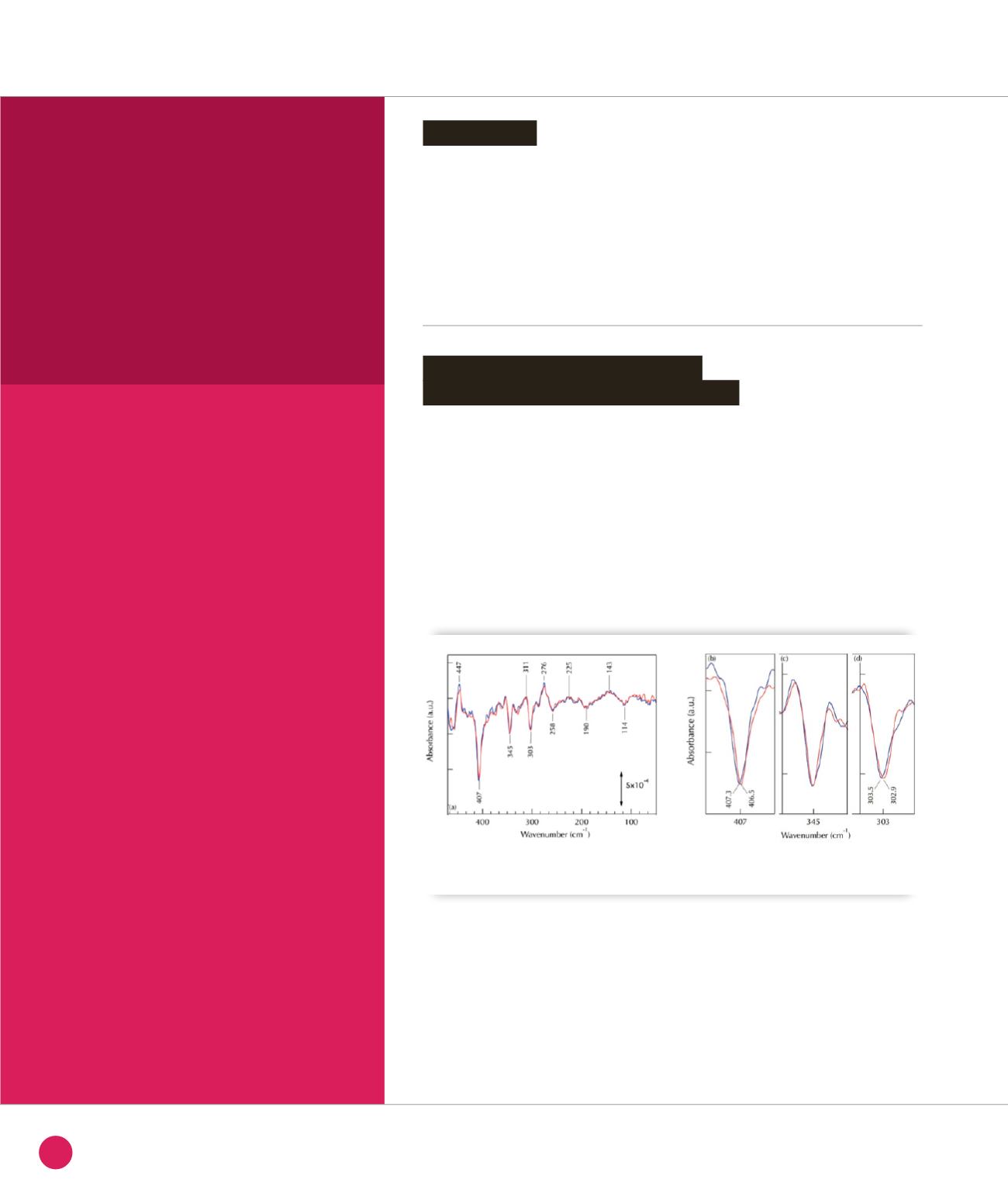
To identify IR modes involving Cu-ligand
vibrations, we compared spectra recorded
with
63
Cu- and
65
Cu-azurin (Figure
➊
).
With
65
Cu, two negative bands at 407
and 303 cm
-1
(CuII state) are downshifted
by 0.8 cm
-1
and 0.6 cm
-1
respectively
(Figure
➊
b
and
➊
d
) suggesting that these
bands correspond to modes involving
CuII-ligand bond. DFT calculations allow
to assign the band at 407.3 cm
-1
to the
ν
(Cu-SCys) mode strongly coupled with
deformation modes of amino acids and
the band at 303 cm
-1
to the
ν
as
(Cu-(NHis)
2
)
IR mode, coupled with bending modes
of the Cu ligands.
Introduction
Identification of metal-ligand IR
modes using metal isotope labelling
CHEMISTRY AND PHYSICAL CHEMISTRY, NANOCHEMISTRY
➊
Effect of
63
Cu- and
65
Cu-azurin isotopic labeling on the reduced-minus-oxidized FTIR difference spectra. (a-d)
Spectra recorded with
63
Cu- (blue) and
65
Cu- (red) azurin.
34
SYNCHROTRON
HIGHLIGHTS
2013


