Lacking crystallographic data for the
peptide and protein complex, the only
indications about the coordination scheme
come from knowledge of the peptide
amino acidic sequence and the preliminary
IR data. We therefore tested in
silico
various geometrical combinations around
the uranyl that were in agreement with
IR, ITC and TRLFS. It consisted of defining
plausible geometries for a first coarse-
grain refinement, using initial interatomic
distances obtained from our previous
work conducted on small phosphorylated
biomolecules resulting in a bidentate
carboxylate and a monodentate phosphate
environment. The next step consisted of
refining all the interatomic distances in the
presence of the peptidic sequence strain
and steric effects using DFT calculations.
Finally, further structural investigation
to probe the uranium coordination
environment was done using EXAFS
spectroscopy. These experiments were
performed at the MARS beamline,
dedicated to the study of radioactive
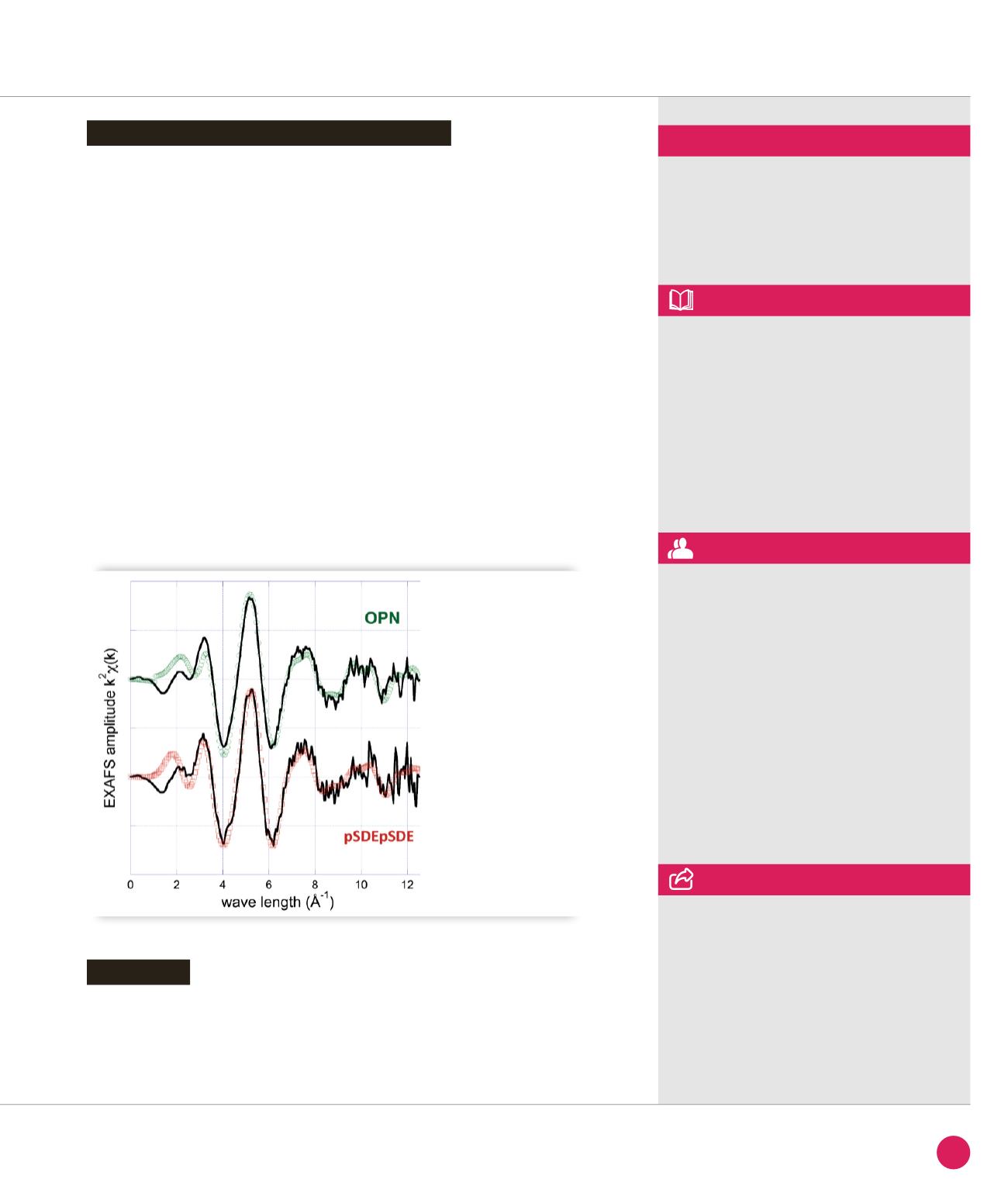
materials. The pSDEpSDE and OPN uranyl
complexes at pH 5.5 exhibit similar EXAFS
pattern as shown in Figure
➋
. Satisfactory
fits obtained for both samples with very
comparable metrical parameters have
been provided. For the two complexes,
axial U-O bond lengths of 1.76 Å were
determined which is typical of a uranyl
aquo ion. The presence of two distinct
equatorial shells at ≈ 2.26 Å and 2.32-
2.33 Å is in agreement with literature data
for phosphate and carboxylate bidentate
bonds respectively. The U–C distance at
2.91-2.93 Å was most consistent with a
four-membered ring chelate resulting from
a bidentate complex with a carboxylate
group. Furthermore the present U–P bond
length (3.83-3.84 Å) is similar to previously
reported for a protonated monodentate
phosphate moiety.
This study has described the uptake of the
uranyl ion by one of the phosphorylated
sites of the OPN protein involved in
bond regeneration. The combination of
several techniques, in particular EXAFS
with synchrotron radiation, has allowed
us to characterize the structural and
thermodynamic factors of uranyl binding to
the OPN site responsible for mineralization
control.
Structure of the uranyl-pSDEpSDE peptide
Conclusion
➋
EXAFS data
of the uranyl-pSDEpSDE
and uranyl-OPN complexes
at pH = 5.5.
Black line = experiment,
circles = fit.
MARS beamline
ASSOCIATED PUBLICATION
Osteopontin: a Uranium phosphorylated
binding-site characterization
S. Safi, G. Creff, A. Jeanson, L. Qi, C. Basset,
J. Roques, P. L. Solari, E. Simoni, C. Vidaud
and C. Den Auwer*
Chem. Eur. J. ; 19(34) (2013) 11261
REFERENCES
[1] Report of the United Nations Scientific
Committee on the Effects of Atomic
Radiation, ISBN 978-92-1-642010-9, 2011
[2] P. W. Durbin, Actinides in animals and man
in The chemistry of the actinide and
transactinide elements, 3
rd
edition
(Eds.: L. R. Morss, N. M. Edelstein, J. Fuger)
Springer 2006
[3] J. D. Van Horn & H. Huang, Coord. Chem.
Rev. 250 (2006), 765
[4] O. Prat et al. Environ. Int. 37 (2011), 657
*Institut de Chimie de Nice,
Université Nice Sophia Antipolis, UMR 7272,
06108 Nice, France
CORRESPONDING AUTHOR
41
SYNCHROTRON
HIGHLIGHTS
2013


