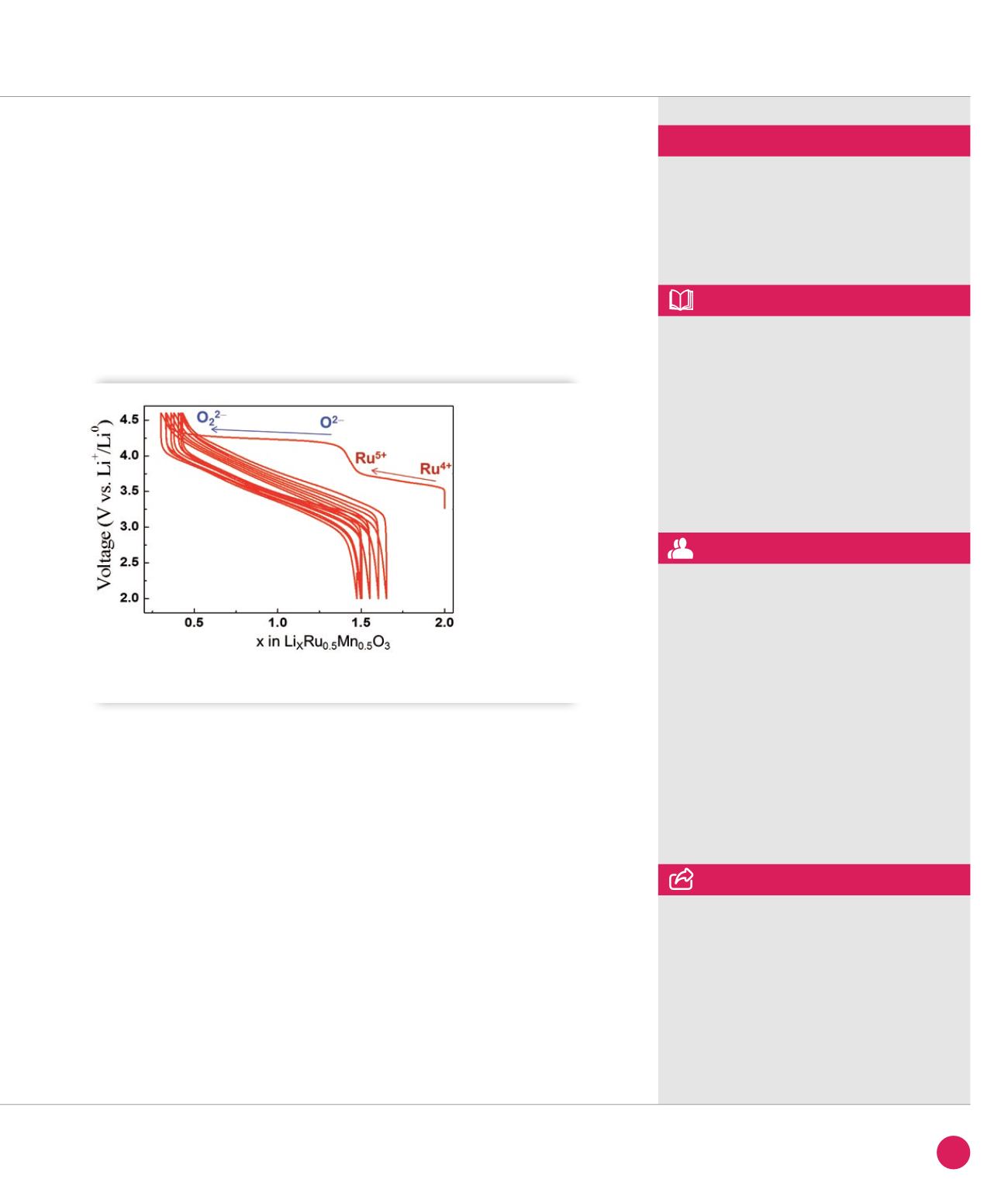
Moreover, we could demonstrate, through
combined
in situ
X-Ray diffraction,
ex situ
Synchrotron diffraction, X-Ray
photoemission spectroscopy and DFT
calculations that the 3.5 V plateau on
charge (Figure
➋
) results from the
classical cationic redox activity (Ru is
oxidized from 4+ to 5+). Nevertheless the
highlight of this paper remains that the
origin of the 4.3 V plateau is nested in an
anionic redox activity which involves the
formation of O
2
2−
peroxo-like groups in
the structure. This result has opened new
research avenues for harvesting novel high
capacity layered electrodes cumulating
within the same structure both cationic
and anionic redox processes [2]. Further
work will include the understanding and
the mastering of the voltage decay these
materials suffer on cycling; let’s bet
chemists will soon provide tricks to avoid
this limitation.
CRISTAL beamline
ASSOCIATED PUBLICATION
High performance Li
2
Ru
1
–yMn
y
O
3
(0.2 ≤ y ≤ 0.8) cathode materials for
rechargeable Lithium-ion batteries:
their understanding
M.Sathiya, K.Ramesha, G. Rousse*, D. Foix,
D. Gonbeau, A. S. Prakash, M. L. Doublet,
K. Hemalatha and J. M. Tarascon
Chemistry of Materials 25 (2013), 1121
REFERENCES
[1] M. Thackeray et al. Journal
of Materials Chemistry 15 (2005), 2257
[2] M. Sathiya et al.
Nature Materials 12 (2013), 827
*Chimie du Solide et Energie,
Collège de France, 11 place Marcelin-Berthelot,
75005 Paris, France
CORRESPONDING AUTHOR
➋
Voltage versus composition curve of Li
2
Ru
0.5
Mn
0.5
O
3
. The redox couple associated with each plateau is indicated.
43
SYNCHROTRON
HIGHLIGHTS
2013


