High capacity Li-rich
layered compounds
for Li-ion batteries
The large capacities displayed by the
Li-rich layered electrodes for Li-ion batteries
have been explored via a thorough study
of the Li
2
(Mn,Ru)O
3
system. Such materials
are based on a staking of Li layers and
honeycomb LiMnRu layers, all cations being
octahedrally coordinated with oxygen atoms.
We could unravel a redox activity linked to
the anionic O
2-
/O
2
2-
couple which comes in
addition to the well-known cationic redox
activity. This fundamental study opens
the path for obtaining batteries lasting
much longer than today’s ones.
Lithium-ion batteries have been
recognized as attractive energy storage
systems not only for portable electronics
but also for powering electric vehicles.
Manganese containing layered compounds
such as LiNi
⅓
Co
⅓
Mn
⅓
O
2
are progressively
replacing LiCoO
2
in today’s Li-ion cells
because of both higher voltage and
capacity. Still, larger capacity values are
needed to meet automobile applications.
Further attempts to improve the
electrochemical properties resulted in
the discovery of new series of composite
cathode materials such as (1-
y
)Li
2
MnO
3
−
y
Li
M
O
2
(
M
= Co, Ni…) which exhibit
higher capacity and excellent stability [1].
Such materials show specific capacity
greater than 200 mAh·g
-1
. However, these
integrated cathodes present complex
layered structures as there is still
ambiguity whether they form short-range
ordered domain or homogeneous solid
solutions. Whatever the exact nature, it
is clear that the high capacity exhibited
by Li
2
MnO
3
− Li
M
O
2
materials cannot be
simply attributed to the presence of two
phases, but is rather more complex.
Our strategy to lift the veil on such a
complexity has been to simplify the
chemical nature of the problem while
preserving a layered structure.
We decided to study the layered
Li
2
Ru
1-y
Mn
y
O
3
system which, in addition
to imparting structural stability, provides
a higher electronic conductivity; then
enhancing the electrode rate capability.
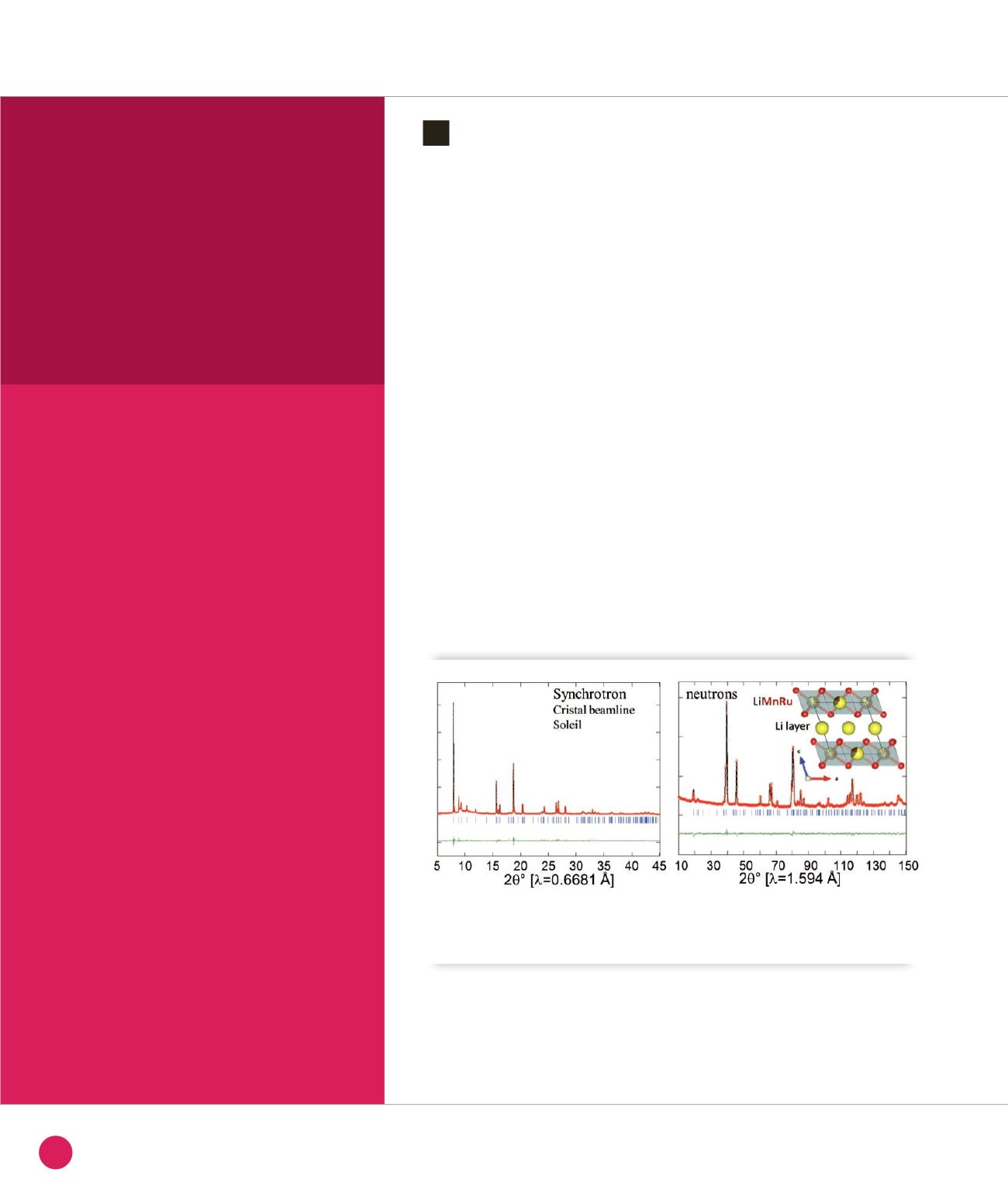
From coupled Synchrotron and neutron
powder diffraction refinements (Figure
➊
),
we could shed light on the distribution of
both light (Li, O) and heavy (Ru, Mn) atoms
in the cell. The resulting analysis clearly
indicates that the sample is single phase
and that the large capacities can therefore
be obtained without having a composite
nature. Li
2
Ru
0,5
Mn
0,5
O
3
is based on a
face-centered cubic stacking of oxygen
atoms, with cations occupying interstitial
octahedral sites so as to generate pure Li
layers alternating with honeycomb LiRuMn
layers.
➊
Rietveld refinements of neutron (D2B, Institut Laue Langevin) and Synchrotron X-Ray diffraction (CRISTAL
beamline) of the Li
2
Ru
0.5
Mn
0.5
O
3
compound, whose structure is shown in inset. The honeycomb LiMnRu layers
alternate with pure Li layers; oxygen atoms (shown as red balls) form a close packed stacking.
CHEMISTRY AND PHYSICAL CHEMISTRY, NANOCHEMISTRY
42
SYNCHROTRON
HIGHLIGHTS
2013


