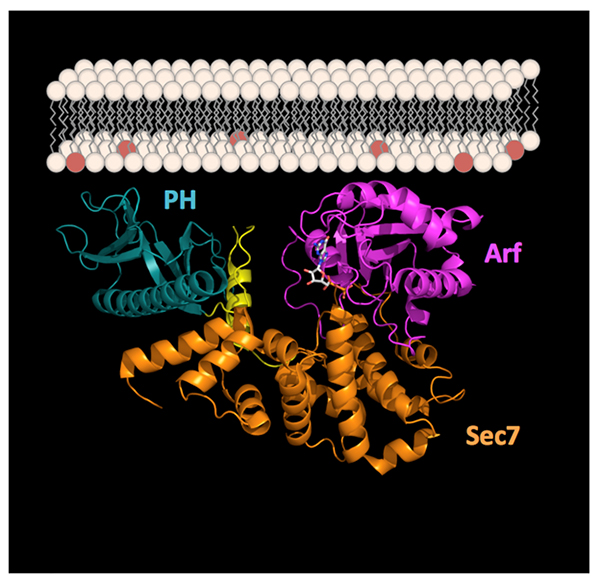
Taking a closer look at small GTPases at the membrane interface
Small GTPases of the Arf family are chief organizers of cellular traffic, the process that targets proteins and lipids to the precise locations in cells where they carry out their functions. The team of Jacqueline Cherfils, at the Laboratoire d’Enzymologie et Biochimie Structurales, CNRS, Gif-sur-Yvette, captured an Arf GTPase in complex with its activator in a conformation that mimics its interaction with membranes, revealing how membranes and regulators team up to juggle activation and targeting.
Small GTPases form a large superfamily that controls many aspects of cell logistics, including membrane and protein traffic, actin cytoskeleton dynamics, and signal transduction. They function as molecular switches by alternating between an inactive state, bound to GDP, and an active state, bound to GTP. Activation of small GTPases by GDP/GTP exchange is tightly regulated by their guanine nucleotide exchange factors (GEFs). Outstanding questions in the small GTPases field is how their regulators, such as GEFs, are themselves regulated, and how this regulation is coupled to membranes and lipids. This study reports the crystal structure of a complex between Arf (a small GTPase that controls most aspects of vesicular traffic in eukaryotic cells), and its GEF BRAG (which activates Arf GTPases in endocytosis and is involved in cancer). The complex was captured in the crystal in a conformation that mimics its interaction at the interface with membranes. Combined with the analysis by fluorescence spectroscopy of the GDP/GTP exchange reaction reconstituted on artificial membranes, the structure reveals how highly efficient activation of Arf GTPases by this GEF is achieved by concurrent optimization of membrane recruitment and nucleotide exchange