Ammonia oxidation – Platinum nanoparticles caught in action
Ammonia oxidation is a key reaction in the chemical industry, essential for global agriculture and mining, and it also helps limit emissions of this irritating and polluting gas. A SOLEIL team, in collaboration with researchers from CEA-Grenoble, used advanced techniques on the SixS beamline to observe in real time how platinum nanoparticles—used as catalysts for this reaction—deform and change shape during oxidation.
By combining surface diffraction and Bragg coherent diffraction imaging (BCDI), the scientists revealed that the size, shape, and internal strain of the particles directly influence their catalytic efficiency and selectivity. Their results, published in Applied Catalysis B: Environmental, deepen our understanding of this reaction and pave the way for the design of more efficient and durable catalysts, with major implications for both industry and the environment.
The oxidation of ammonia (NH₃) is a vital industrial process for producing nitric oxide (NO), an essential intermediate in the manufacture of nitric acid (HNO₃)—used in fertilizers, explosives, and dyes. However, ammonia oxidation does not produce NO alone; it also generates nitrous oxide (N₂O) and nitrogen gas (N₂). All three products have industrial relevance, and the challenge lies in maximizing the yield of one or the other—this is known as Selective Catalytic Oxidation.
For over a century, platinum (Pt) has been the reference catalyst for NO production. Interestingly, it is used in the form of micrometric wires woven into metallic gauzes rather than as dispersed nanoparticles—one of the last remaining examples of bulk solid catalysts in industrial use. These wires are metallic and polycrystalline, composed of grains similar in size to the particles studied here. Yet, despite more than a hundred years of research, the precise mechanisms by which platinum particles or crystals influence the selectivity and efficiency of the reaction remain only partially understood, particularly under real operating conditions (high temperature, pressure, and gas mixtures).
Results and insights from the study
For the first time, a team of researchers used a unique operando coupling of techniques—allowing observation under actual reaction conditions—on SOLEIL’s SixS beamline: Surface X-ray Diffraction (SXRD) and Bragg Coherent Diffraction Imaging (BCDI). Together, these methods made it possible to monitor both the average structural behavior of an ensemble of particles and the deformation of individual particles during ammonia oxidation.
The results show that:
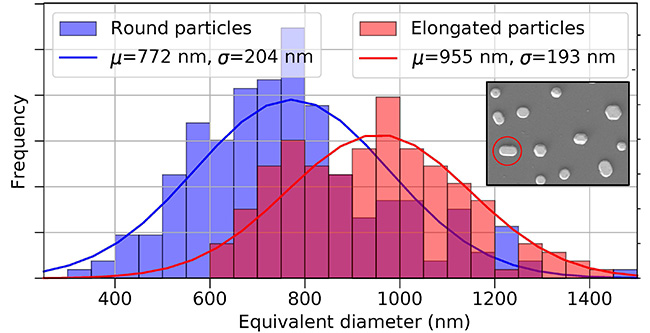
Platinum particles of different sizes and shapes (elongated or round, Figure 1) behave differently under varying temperatures and reaction gas compositions.
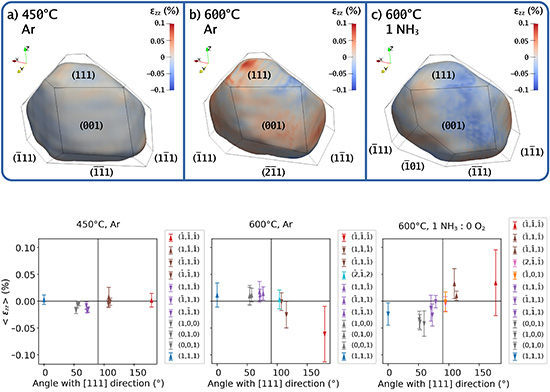
Internal mechanical strains depend on both particle size and their interaction with the alumina (Al₂O₃) support. For instance, elongated particles—subjected to stronger stresses—undergo reversible structural changes in the presence of ammonia (Figure 2), whereas round particles develop crystalline defects at high temperature.
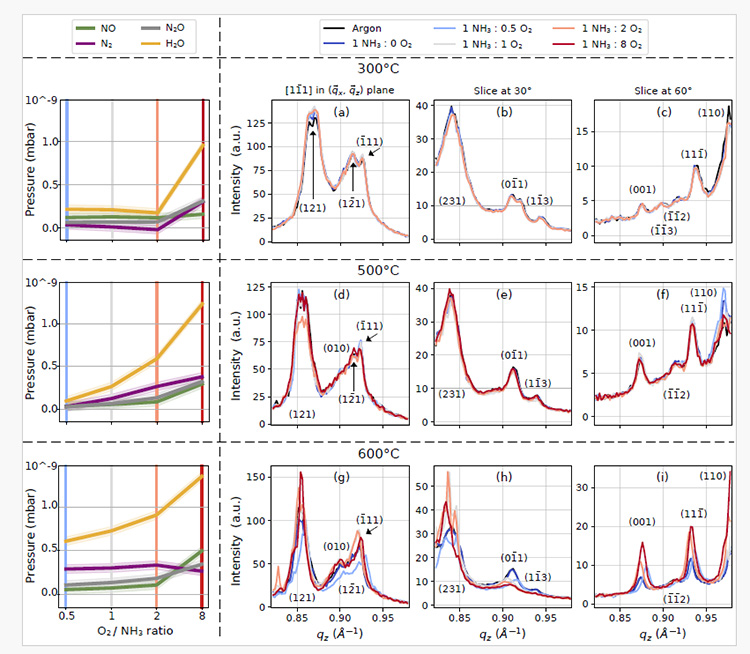
The reaction selectivity (NO vs N₂ or N₂O production) is directly linked to these morphological changes. An increase in the partial pressure of oxygen promotes NO formation (Figure 3), and the most strained Pt particles appear to be more stable and selective under these conditions.
SOLEIL’s contribution
This study was made possible thanks to the dedicated catalytic reactor and advanced optics of the SixS beamline, which can focus the X-ray beam down to the sub-micrometer scale while maintaining high coherence. These unique experimental conditions enabled real-time monitoring of the structural transformations of the nanoparticles.
Moreover, the combined use of SXRD (to study ensembles of nanoparticles) and BCDI (to analyze individual particles) revealed behavioral differences that would remain invisible with a single technique. For example, BCDI showed that elongated nanoparticles in close contact with the substrate accommodate strain asymmetrically, affecting their catalytic activity.
Outlook
These findings open new avenues for optimizing industrial catalysts:
- Tailored nanoparticle design – by controlling particle size, shape, and internal strain, it will be possible to engineer more selective and stable catalysts.
- Development of bimetallic catalysts – platinum–rhodium (Pt–Rh) alloys, already used in industry, could be further optimized by exploiting the strain effects observed in this study.
- Understanding degradation mechanisms – insights into how crystalline defects form and evolve will help extend catalyst lifetimes.
In the longer term, these advances could contribute to cleaner and more efficient industrial processes, aligned with global sustainability and ecological transition goals.
This project has received funding from the European Research Council (ERC) under the European Union’s Horizon 2020 research and innovation programme under Grant Agreement No. 818823.