In situ study of the mechanisms of atmospheric corrosion of iron
It is crucial to be able to study corrosion and predict the behavior of metals over long periods, especially in the specific context of managing the conservation of cultural heritage materials. The conditions of this atmospheric corrosion have been recreated in an electrochemical cell and studied on the DIFFABS beamline.
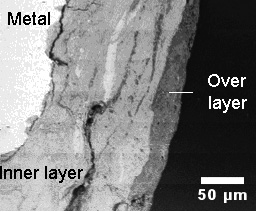
The mechanism of atmospheric corrosion of iron in sheltered structures over the very long term follows a wet - dry cycle. During this cycle and in parallel with iron oxidation, reduction of the layer of corrosion products takes place in between the healthy metallic core and the atmosphere (Figure 1).
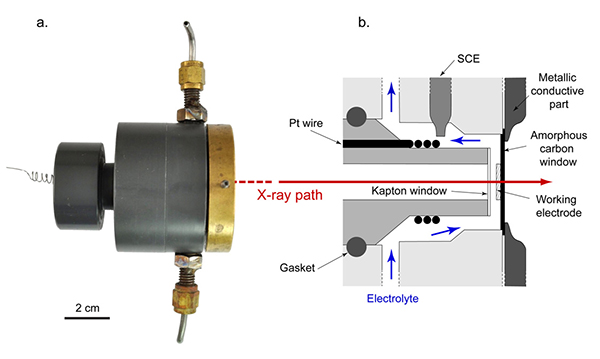
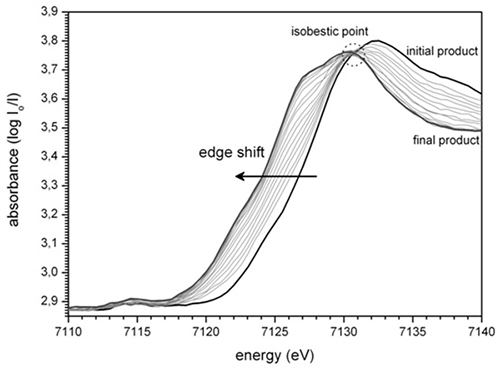
By recreating the conditions of this atmospheric corrosion in an electrochemical cell (Figure 2), it was possible to follow in situ the reduction mechanism of certain phases of oxidized iron. The process was followed for two phases, the lepidocrocite and ferrihydrite, with two complementary techniques, X-ray diffraction and X-ray absorption spectroscopy, which respectively provided information on the crystal and local structures around the iron.
The experiments were performed on the CRG FAME beamline at ESRF and on the DIFFABS beamline at SOLEIL. It was possible to identify the (magnetite and ferrihydrite) phases that are formed during reduction, thus improving our understanding of very long-term iron corrosion mechanisms.
This research on the above model systems was done in parallel with studies on iron corrosion products from ancient materials (iron used in historical monuments or museum objects), conducted in particular by the Archaeomaterials and Alteration Prediction Laboratory (LAPA, UMR CEA-CNRS).