Iron-rich (bio)minerals: Transport of iron, carbon and trace elements from Icelandic wetlands
In streambeds of freshwater wetlands, accumulations of bright orange-brown ‘flocs’ are commonly seen.
To better understand their role in diverse biogeochemical cycles, the Soil Chemistry Group (ETH Zurich) characterized flocs from freshwater wetlands in Iceland. Using X ray absorption spectroscopy data collected at the SAMBA beamline, they aimed to identify the Fe(III) minerals present in the flocs and sought to assess the extent and nature of trace element sorption to the floc Fe(III) minerals.
As the color would suggest, these flocs are often rich in various ferric Fe (Fe(III)) minerals. In general, Fe(III) minerals have high surface areas to which nutrients, carbon and trace elements sorb. Thus, flocs, and the Fe(III) minerals they comprise, are important players in the biogeochemical cycling of nutrients, carbon and trace elements in freshwater wetlands.
In subsurface layers of freshwater wetlands, oxygen becomes limited, leading to the establishment of anoxic conditions. Under anoxic conditions, Fe(III) minerals are reduced through microbial processes to Fe(II). Associated compounds, including nutrients, carbon, and trace elements, are then solubilized and are transported through the groundwater in the aqueous phase. Depending on the groundwater flow trajectory, this oxygen-poor groundwater may mix with oxygen-rich surface waters, often in streambeds of wetland surface streams (Fig. 1).
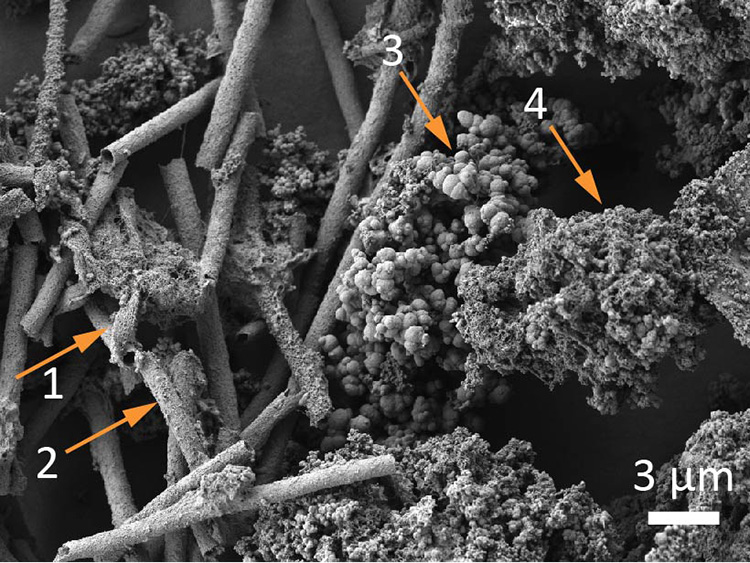
At this mixing interface, exposure of Fe(II) to atmospheric oxygen leads to rapid oxidation of Fe(II) to Fe(III) and precipitation of orange-brown colored Fe(III) minerals. Over time, the precipitated Fe(III) minerals aggregate, forming complex associations of mineral phases, microorganisms, and organic detritus that are commonly referred to as ‘flocs’ (Fig. 2).
The sorption of nutrients, carbon and trace elements to Fe(III) minerals can alter their bioavailability, speciation, and mobility. Floc-Fe(III) minerals tend form rapidly (upon exposure of Fe(II) to atmospheric oxygen), are often poorly-crystalline and characterized by high mineral surface areas. This leads to a high potential for sorption of compounds and thus impacts the biogeochemical cycling of these various compounds in wetland environments. Moreover, flocs are easily mobilized; increases in stream flow rates (wind, rain events) can mobilize and transport the flocs -and associated compounds- further downstream, and may eventually contribute to their export out of wetland environments.
To quantitatively assess the speciation of Fe in the flocs, the scientists collected X-ray absorption spectroscopy data (XANES and EXAFS) at the SAMBA beamline. Results showed that the majority of floc-Fe was found in ferrihydrite, a poorly-crystalline Fe mineral. Smaller fractions of Fe were found in crystalline minerals (lepidocrocite and goethite); the fraction of Fe in clay minerals was minor.
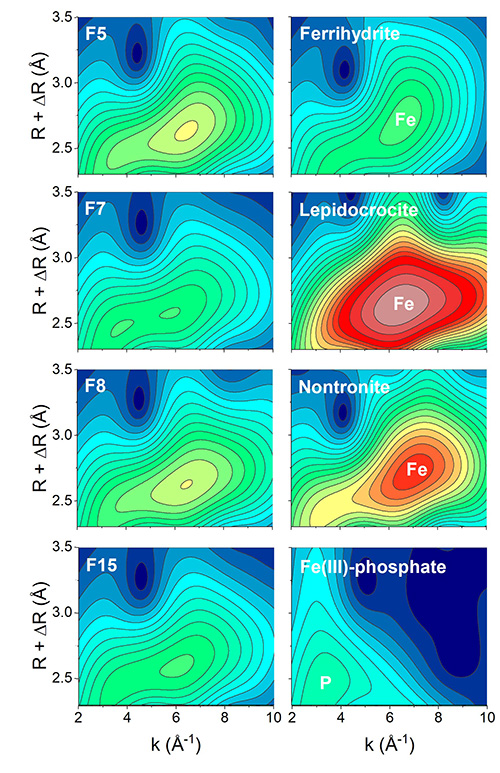
Additionally, Morlet wavelet transform analyses* were conducted on the collected EXAFS spectra. Thus, the scientists qualitatively identified significant fractions of organic- and P-complexed Fe (Fig. 3). In the laboratory, they also determined the total trace element content of the flocs and assessed in which fraction (poorly-crystalline or crystalline) the trace elements were found. Results showed that nearly all trace elements in the flocs were associated to poorly-crystalline mineral phases, which, based on the X-ray absorption spectroscopy data, is most likely the ferrihydrite.
Conclusions
The primary Fe(III) mineral in the Iceland flocs is ferrihydrite and the majority of trace elements in the flocs are found sorbed to this ferrihydrite fraction. Thus, the poorly-crystalline Fe(III) minerals in the Iceland flocs effectively sequester trace elements. However, the flocs are easily mobilized, and thus likely act as transport vectors for nutrients, carbon, trace elements, and Fe leaving the wetland. Ferrihydrite is also prone to reductive dissolution. Considering that flocs may eventually be exposed to varying geochemical conditions, the high fraction of ferrihydrite-associated trace elements suggests that the flocs likely lack long-term trace element storage capacity.
* These analyses not only provide information on the distances between the atom of interest (here, iron) and neighbouring atoms - information already available from Fourier Transformation of the EXAFS spectra - but also allow to discriminate between several neighbouring atoms located at the same distance from the atom of interest - information that is not available from Fourier Transformation of the EXAFS.