Imaging Alzheimer’s disease through three different beamlines at SOLEIL
Alzheimer’s disease — the most common dementia in the world — progresses in the brain with the development of large protein deposits called amyloid-beta plaques. These shell-like objects can entrap metals that are involved in the activity of the brain.
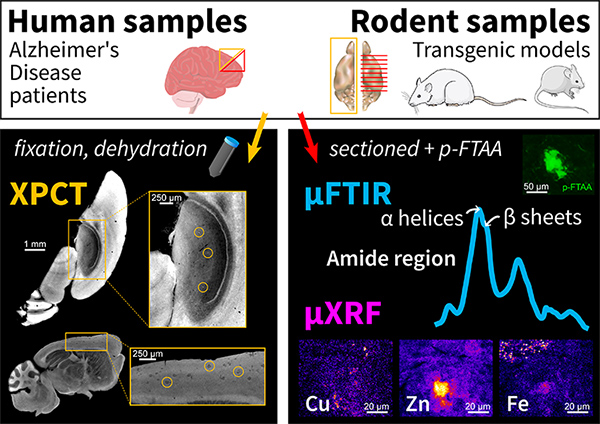
Researchers at the “Centre de Recherche en Neurosciences de Lyon” (CRNL) and the “Synchrotron Radiation for Biomedicine” group (STROBE, Grenoble) have used a 3D imaging method, X-ray phase-contrast tomography (XPCT), to study samples of brains affected by the disease. Using intense X-rays produced by the synchrotron, XPCT is an important advance on visible-light microscopy, which only provides 2D images and requires the clinician to slice the samples. Their new study involves 3 SOLEIL beamlines. It reveals that XPCT can detect amyloid-beta plaques thanks to the metals they entrap.
While Alzheimer’s disease affects one in ten persons over 65 years of age worldwide, its diagnosis is only confirmed upon autopsy: the clinician selects a few areas of the brain that are sliced and analyzed with a microscope. New high-resolution 3D techniques are arising to simplify the diagnosis at death. Among them, X-ray phase-contrast tomography (XPCT) can detect the amyloid-beta plaques, which are a marker of the disease. Researchers from the Lyon Neuroscience Research Center (CRNL) in Lyon and from the Synchrotron Radiation for Biomedicine group (STROBE) in Grenoble have conducted a study to understand what is detected in the plaques by this tomography technique.
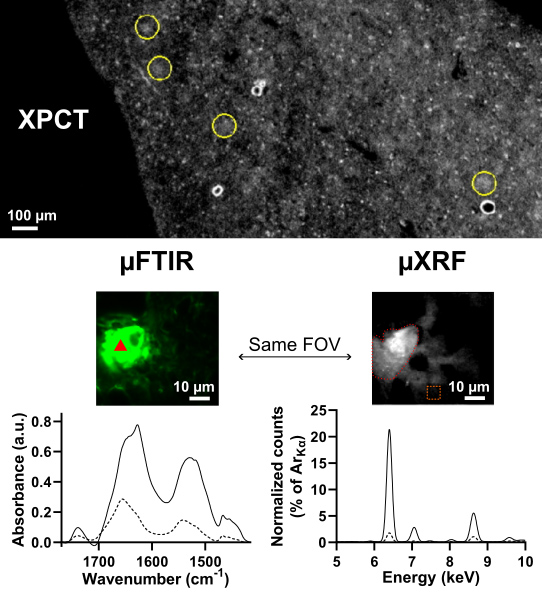
This study started with two hypotheses: XPCT detects (1) the aggregated proteins (fibrils) that compose the plaques or (2) the metals that get captured in the plaques. To find out which of these assumptions is correct, samples were scanned by XPCT on the ANATOMIX beamline and then by two spectroscopy techniques, also at SOLEIL: Fourier transform infrared microspectroscopy (µFTIR) on the SMIS beamline, and X-ray microfluorescence spectroscopy (µXRF) on the NANOSCOPIUM beamline. µFTIR detects the characteristic "signatures" of large molecules such as proteins so it was used to test the first hypothesis, while µXRF gives access to the chemical composition of the samples studied and was used to test the second.
Comparison of the XPCT data with those obtained using the other two techniques showed that the more metals were concentrated in the plaques (especially iron, copper and zinc), the brighter the plaques appeared in the images from XPCT; on the contrary, the overall quantity of fibrillar proteins was similar in all the animal models studied and did not on its own explain the differences observed in XPCT.
Besides, this study unveiled strong differences in the metal accumulation and protein aggregation, first between familial and non-familial cases of Alzheimer’s disease: deposits from a familial case contained more metals and more fibrils; and second between human samples and animal models of Alzheimer's disease: deposits were larger, and metals were more spread out in human samples.
Future work could use the full 3D capabilities of the XPCT technique in a cohort study, to compare this "virtual histology" with standard histological techniques, which require tedious slicing and marking on microscope slides.
|
Film: 3D rendering of the brain (courtesy of brain-map.org) showing the frontal cortex, followed by 3D rendering of the scanned sample from the genetic Alzheimer's disease patient and browsing of the annotated virtual slices of the same sample. Resolution: 3.09µm. |
Acknowledgements: the authors would like to thank the scientists at SOLEIL beamlines for their support, as well as the donor patients and their families.