ANATOMIX

The beamline ANATOMIX (Advanced Nanotomography and Imaging with coherent X rays) works at photon energies between 5 and 50 keV. It is dedicated to full-field radiography and tomography in absorption and phase contrast, with pixel sizes from 20 nm to 20 µm.
ANATOMIX is a beamline for X-ray tomography on the micro- and nanoscale, in absorption and phase contrast. It operates in the energy range from 5 keV to more than 50 keV and allows its users to obtain two- and three-dimensional radiographic images of bulk volume samples of macroscopic size (up to several cm thickness). For smaller samples, a spatial resolution down to 50 nm (20 nm pixel size) can be achieved. Real-time studies are possible at speeds of currently up to one microtomography scan per second; higher speeds up to 20 volume scans per second (50 ms per scan) have been demonstrated.
A flexible sample interface enables in situ and/or operando studies under conditions similar to the natural or working environment of the samples (temperature, humidity, mechanical load, transport processes). Biological samples can be measured without dehydration and, in many cases, without chemical fixation. With suitable sample preparation, cellular imaging without cryogenic environment is possible.
Like all other SOLEIL beamlines, ANATOMIX is open to users from all countries, free of charge when applying for beamtime via a proposal. If you are considering measurements on ANATOMIX, be sure to check out our Beamline User Guide (PDF format) and contact the beamline staff to discuss your project ("Contacts" section below).
| ANATOMIX is an Equipment of Excellence (EQUIPEX) funded by the Investments for the Future program of the French National Research Agency (ANR), project NanoimagesX, grant no. ANR-11-EQPX-0031. |
Experimental techniques on ANATOMIX
Recent papers with results from the beamline:
|
Contacts
Want to perform an experiment on ANATOMIX? Or find out whether the beamline might be suited for your project? The ANATOMIX beamline team members are happy to answer your questions and give advice:
| Timm Weitkamp Scientist in Charge | +33 (0)1 69 35 81 37 timm.weitkamp@synchrotron-soleil.fr |
| Mario Scheel Scientist, project leader nanotomography (TXM) | +33 (0)1 69 35 96 31 mario.scheel@synchrotron-soleil.fr |
| Jonathan Perrin Scientist | +33 (0)1 69 35 96 59 jonathan.perrin@synchrotron-soleil.fr |
| Guillaume Daniel Technician | +33 (0)1 69 35 96 66 guillaume.daniel@synchrotron-soleil.fr |
| Hubert Chevreau Industrial liaison scientist | +33 (0)1 69 35 97 00 hubert.chevreau@synchrotron-soleil.fr |
| Alessia Melelli Postdoctoral researcher | +33 (0)1 69 35 96 59 alessia.melelli@synchrotron-soleil.fr |
| Shyam Pulickan Postdoctoral researcher | +33 (0)1 69 35 94 96 shyam.pulickan@synchrotron-soleil.fr |
Telephone numbers of beamline rooms
| Beamline control room near optics hutches | +33 (0)1 69 35 97 31 |
| Beamline control room experiments | |
| Experiment hutch EH3 | +33 (0)1 69 35 97 82 |
| Experiment hutch EH4 | |
| Meeting and data analysis room | +33 (0)1 69 35 97 71 |
| Preparation laboratory | |
| Workshop | +33 (0)1 69 35 99 80 |
Beamline news
All beamline news
SOLEIL Highlights 2024

SOLEIL's contribution to the Energy Transition
Team
* External service provider, temporary or collaborator
Employment
Click here to access the SOLEIL employment web page
Technical data
- Experimental techniques
-
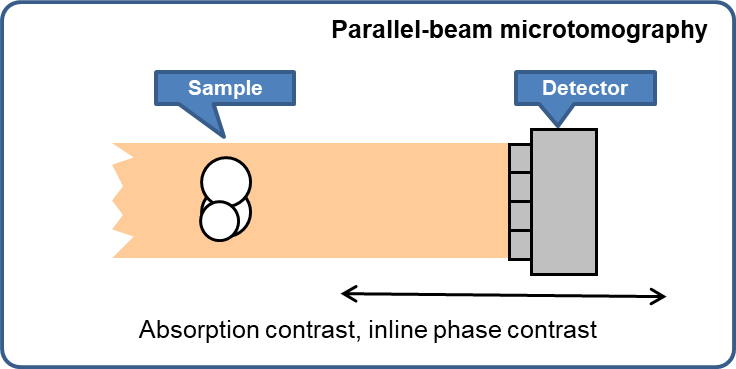
Parallel-beam full-field microtomography
- Absorption contrast
- Inline phase contrast
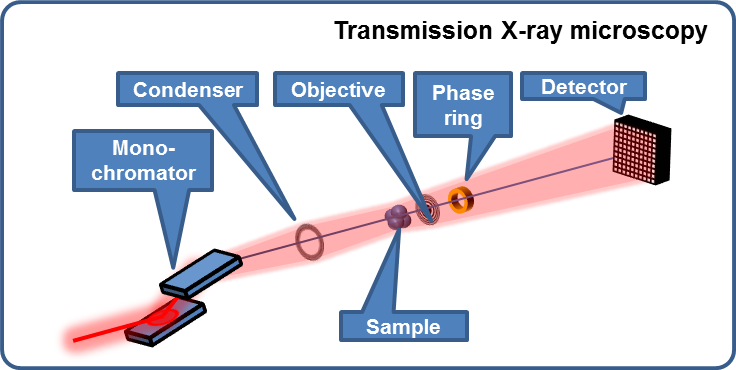
Nanotomography via full-field zone-plate microscopy (= transmission X-ray microscopy, TXM)
- Absorption contrast
- Zernike phase contrast
- Energy range
-
Microtomography:
- Between 5 and 50 keV (white beam)
- Up to approximately 25 keV (monochromatic)
Nanotomography:
- 5 to 11 keV, 17 keV (monochromatic)
- Beam size at sample
-
Microtomography:
- maximum approx. 20 mm (H) × 15 mm (V)
- with mirror M1-M2: maximum approx. 40 mm (H) × 15 mm (V)
Nanotomography:
- Approximately 0.04 mm × 0.04 mm
- Beam modes / energy resolution
-
Microtomography:
- Filtered white beam
- Double crystal monochromator Si-111: ΔE/E = 10-4
- Double multilayer monochromator: ΔE/E = 10-2 (planned)Nanotomography:
- Double crystal monochromator Si-111: ΔE/E = 10-4
- Source
-
U18 cryogenic in-vacuum undulator
- X-ray optics
-
Entrance aperture: Diaphragm 2.5 mm × 2.0 mm (H×V), 22.7 m from source.
Horizontal "coherence" slit, 23.2 m from source.
Primary slits, 26 m from source.
Double mirror (removable), horizontal reflection, horizontally focusing, f=3.5 m, 35.5 m from source.
Refractive lenses for collimation (removable), 38 m from source (planned).
Secondary horizontal source slit (for use with mirror), 39 m from source.
Double crystal monochromator (Si-111, removable), vertical deflection, 50 m from source.
Double multilayer monochromator (removable), vertical deflection, 53 m from source (planned).
- Detectors
-
All detectors are indirect, lens-coupled systems: the X-ray image is converted into a visible-light image by a scintillator, then projected onto a digital pixel sensor by visible-light lens optics. In microtomography, the effective pixel size is the sensor pixel size divided by the magnification factor of the detector optics. For example, using a detector optics set with a magnification ×10 with a camera whose sensor pixel size is 6.5 µm will result in an effective pixel size of 0.65 µm. (In TXM nanotomography, this value has to be further divided by the X-ray magnification factor of the X-ray microscope to obtain the pixel size at sample level.)
Detector optics:
Available magnifications: ×0.48, ×1, ×2.1, ×5, ×7.5, ×10, ×20, ×50 Sensors:
Model Orca Flash 4.0 V2 Orca Lightning Dhyana95 V2 Dimax HS4 Manufacturer Hamamatsu Hamamatsu Tucsen PCO Sensor type CMOS CMOS CMOS CMOS Sensor array size 2048×2048 4608×2592(a) 2048×2048 2000×2000 Sensor pixel size 6.5 µm 5.5 µm 11.0 µm 11.0 µm Max. frame rate(b) 20 fps(c) 30 fps 24 fps 2277 fps Exposure time per frame 40 µs to 10 s 50.4 µs to 1 s 21 µs to 10 s 1 µs to 40 ms Frame buffer size ≈1 TB(d) ≈1 TB(d) ≈1 TB(d) 36 GB(e) ADC bit depth 16 bit 16 bit 16 bit 12 bit Max. SNR 37000 17000 31600 1600 Peak quantum efficiency 82 % 60 % 95 % 47 % (a)Camera is always mounted in landscape orientation. We recommend limiting the ROI to no more than 3000×2400 pixels to avoid distortion effects. (b)For a full, unbinned frame. (c)Supplier specifies up to 100 fps but we encounter stability issues above 20 fps. (d)Limited by hard-disk size of camera PC. (e)Limited by camera on-board memory.
Scientific opportunities
| Information accessible |
|
|---|---|
| Functional studies |
|
| Application areas |
|
Examples: Parallel-beam microtomography
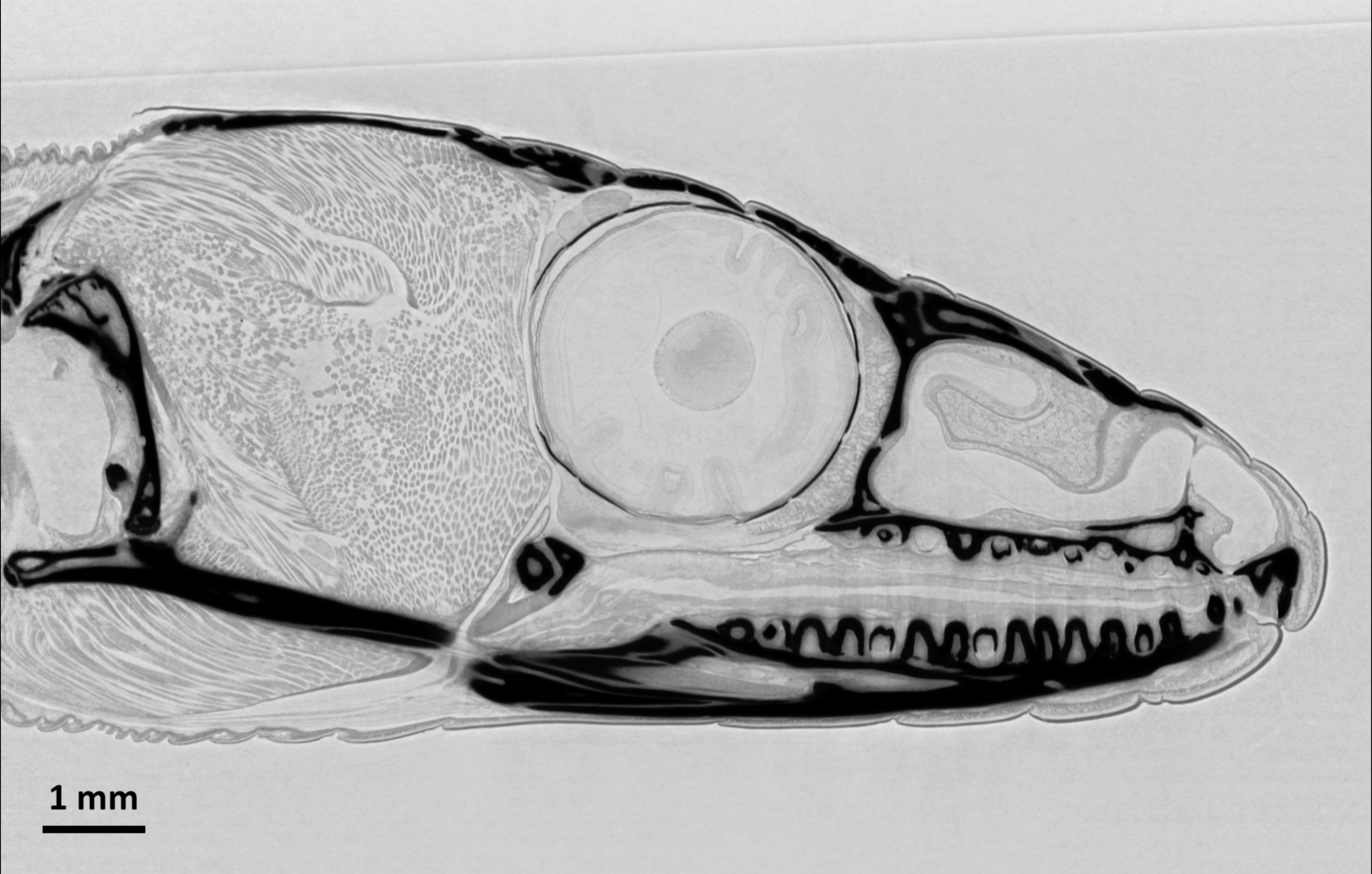
| Microtomography with a wide beam: Tomographic slice through the head of a common wall lizard (Podarcis muralis). Note the soft tissue details, visible through X-ray phase contrast. The dead animal was kept in ethanol for the measurements. Data acquired on ANATOMIX using an indirect detector (scintillator LuAG, 1× photo-objective optics and a scientific CMOS camera) with an effective pixel size of 6.5 µm, resulting in a spatial resolution around 15 µm. The volume data set was collected in 7 minutes using a filtered white X-ray beam with a central energy around 25 keV, at a sample–detector distance of 1.2 m. |
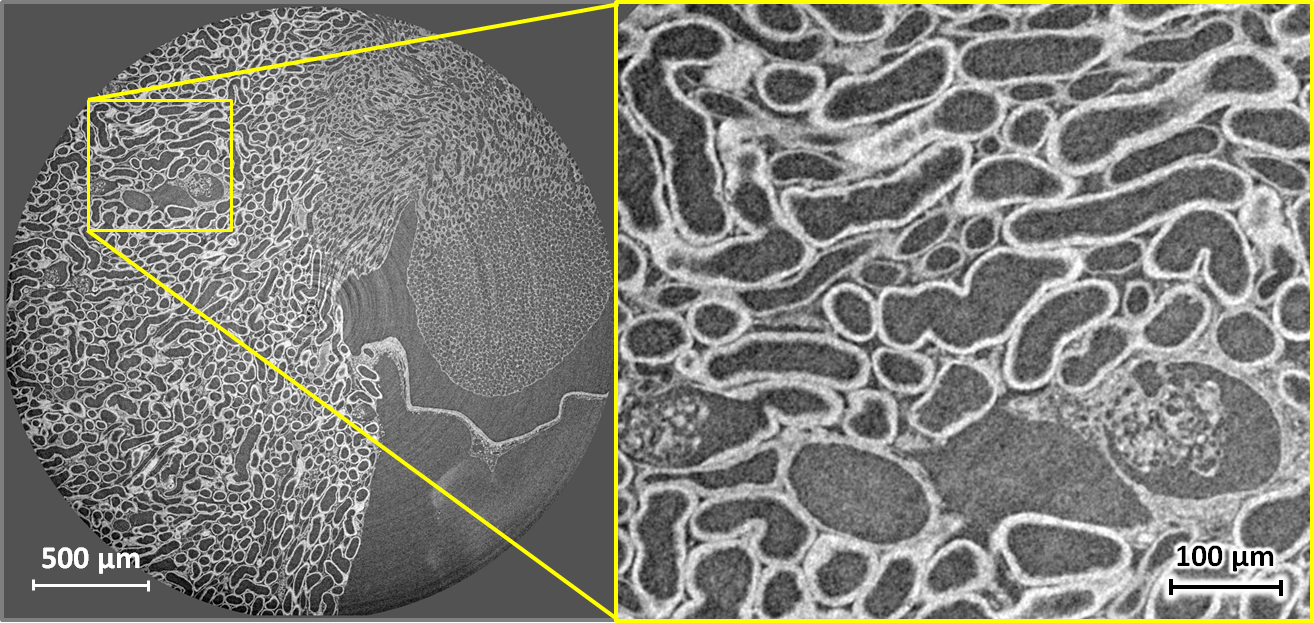
| Tomography of stained soft tissue: Horizontal X-ray phase-contrast microtomography slice through the kidney of a mouse. The full volume was recorded in 5 minutes at 17 keV and a sample–detector distance of 33 mm, using an indirect detector (scintillator, 5× microscope objective and a scientific CMOS camera) with an effective pixel size of 1.3 µm, resulting in a spatial resolution around 2.5 µm. Phase retrieval by Paganin filtering was applied to obtain the images shown. The specimen was prepared using 90 mg/ml iodine contrast agent in 2% agar. Left: full slice. Right: enlarged detail of the left image. Sample courtesy Georg Schulz, Biomaterials Science Center, University of Basel, Switzerland. |
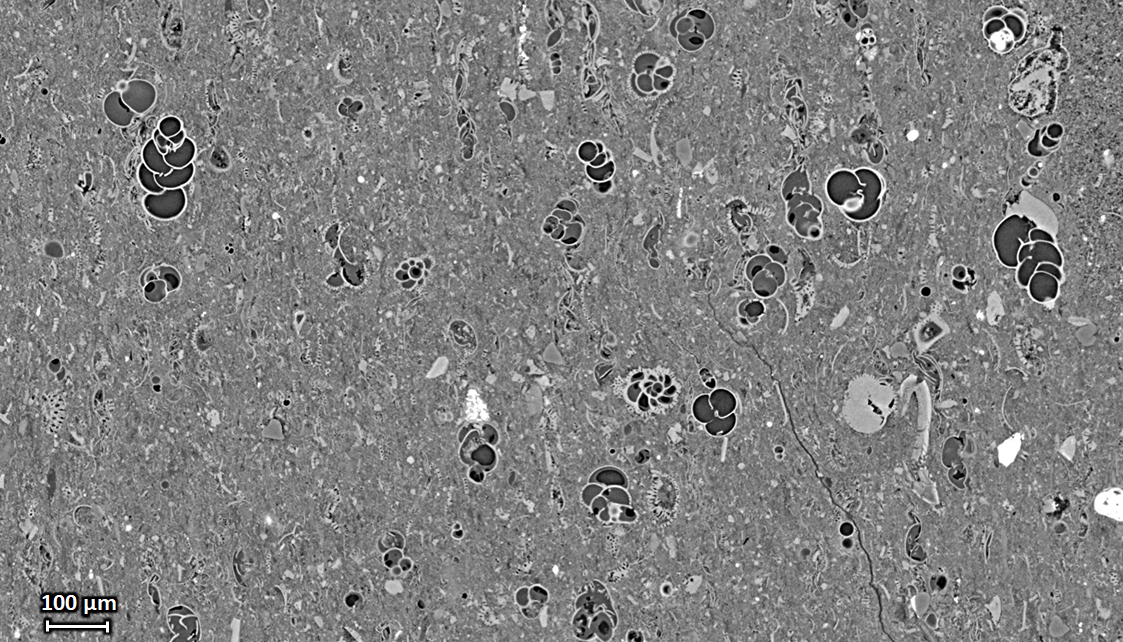
| Extended-field microtomography: Vertical tomography slice through a piece of shale rock with foraminifera inclusions, acquired with a pixel size of 0.65 µm. The field of view was extended by positioning the rotation axis away from the center of the detector field of view. Sample courtesy R. Pellenq, MSE², umi.mit.edu, Massachusetts Institute of Technology / CNRS / Aix-Marseille Université, Cambridge, Massachusetts, USA. |
Examples: Transmission X-ray microscopy
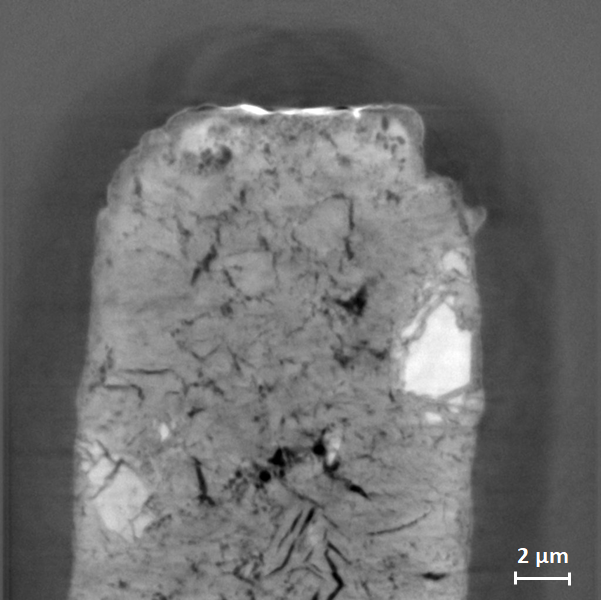
| Nanotomography slice of a sample of cement paste at 10 keV. The TXM was used in Zernike phase contrast at a pixel size of 21 nm. The estimated resolution is 85 nm (H) × 75 nm (V). The sample cylinder was prepared with FIB-SEM (i.e., focused-ion-beam milling combined with scanning electron microscopy). Sample courtesy R. Pellenq, MSE², umi.mit.edu, Massachusetts Institute of Technology / CNRS / Aix-Marseille Université, Cambridge, Massachusetts, USA. |
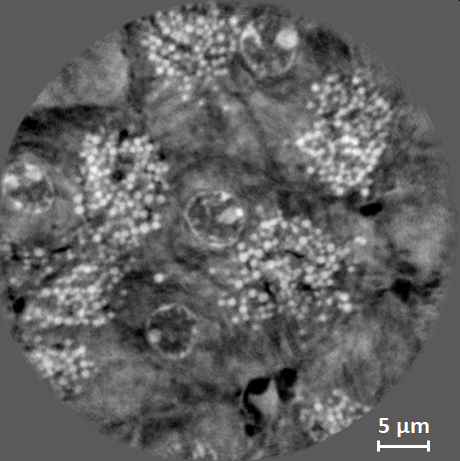
| Local nanotomography of soft biological tissue, here: a murine pancreas at 10 keV. The image shows an axial slice through the reconstructed volume. The TXM was used in Zernike phase contrast at a pixel size of 44 nm. The estimated resolution is about 200 nm. The total exposure time for the scan was 133 minutes. The pancreas was extracted, fixed in formol, then dehydrated and included in paraffin. The sample was cut into a cube column of 1.5 mm diameter and a volume of 40 µm diameter and 40 µm height was imaged in local tomography. Sample courtesy Raphael Scharfmann, INSERM / Institut Cochin, Paris, France. |