Finding alternatives to opioids : Nanoparticles of analgesic prodrugs studied on SWING and DISCO
A major global health challenge nowadays is the opioid crisis. Opioids are the most powerful and widely used painkillers with however severe addiction drawbacks. In this context, a ground-breaking analgesic nano-medicine approach was proposed by the Institut Galien, based on prodrugs* consisting of a molecule with analgesic action linked to a protective chemical group, which spontaneously self-assemble into nanoparticles.
Through collaborative research, scientists from the Institut Galien Paris-Saclay and the NIMBE (CEA/CNRS) have investigated the physico-chemical features of 3 prodrugs with different analgesic profiles. They have discovered that changing the linker between the drug and the lipid moiety induced significant differences in the structure of the nanoparticles and in their interaction with albumin, a model blood protein.
Nanotechnology represents a very smart approach for targeted delivery, controlled release, and protection from rapid metabolization of drugs. However, if the research in the field of nanomedicines has been very active in the last 20 years, bringing unexpected breakthroughs (the COVID vaccine not being the least), the translation from the bench to the clinic remains challenging. Indeed, despite their proven efficacy, several aspects of the mechanism of action of drugs remain poorly understood.
It’s notably the case for prodrugs such as LENK-SQ whose analgesic efficacy is difficult to predict. LENK-SQ is the result of the coupling of an active molecule: leu-enkephalin "LENK", an endogenous neuropeptide known to be rapidly degraded and metabolized, and squalene "SQ", a natural and biocompatible lipid. The LENK-SQ bioconjugates exhibit the ability to self-assemble spontaneously in water to form nanoparticles (NPs). The squalene-based NPs allow protection of the LENK drug, allowing a better targeting in vivo, thus enhancing the pharmacological activity and reducing the toxicity and side effects of the LENK.
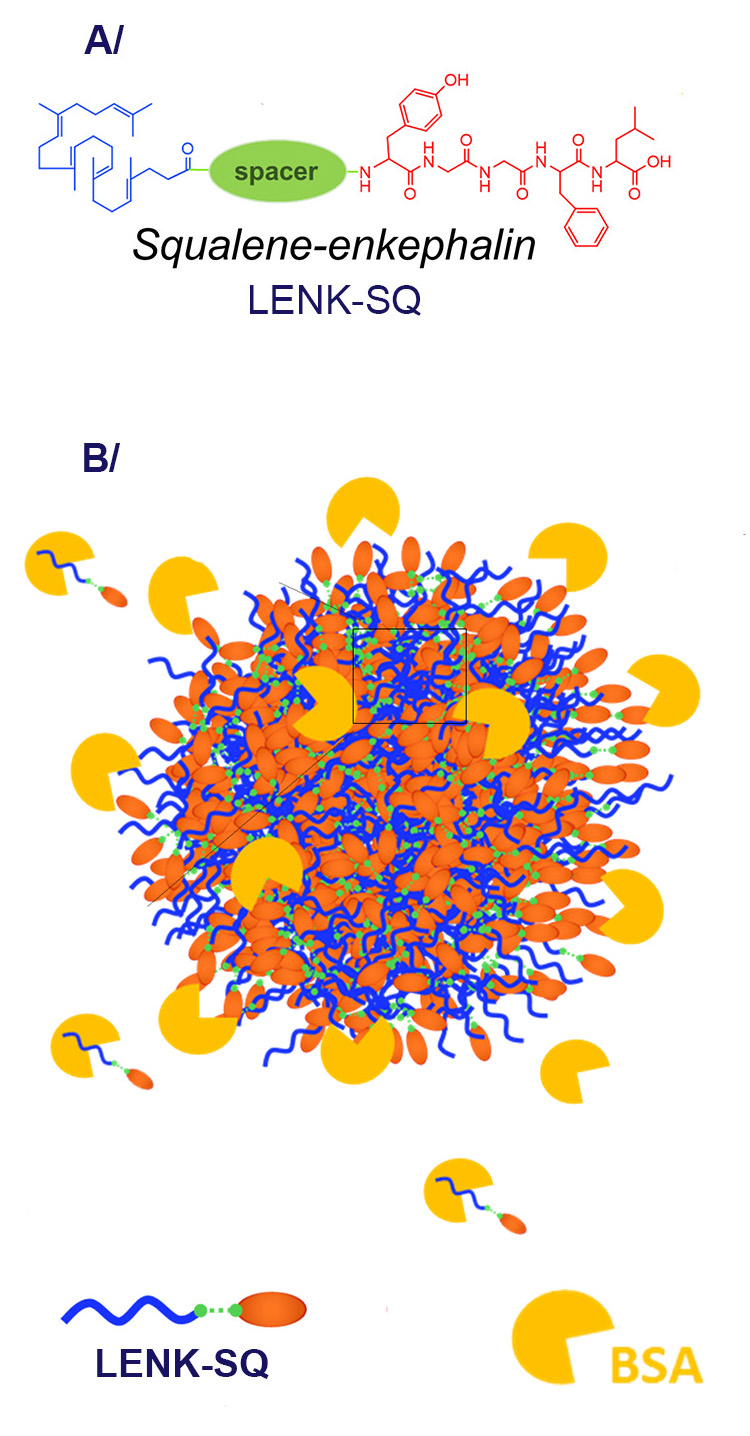
Scientists at the Institut Galien Paris-Saclay obtained three different LENK-SQ bioconjugates by modifying the chemical grouping ("spacer" in Figure 1) linking the neuropeptide and squalene, a spacer labelled Am, Dig or Acyl, in order to modulate the release and analgesic profiles of Leu-enkephalin.
While the administration of LENK-SQ bioconjugates in the form of NPs exhibited promising results, an important aspect which remained poorly understood was the variability of analgesic profiles among the three types of LENK-SQ nano-assemblies. These unexpected results were difficult to explain and were not linked to purely molecular considerations.
Researchers from the Institut Galien Paris-Saclay and the NIMBE posit that a better understanding of the physico-chemical properties of these complex nanoobjects could help to better predict and understand their in vivo behaviour and also highlight some weak points (such as formulation reproducibility, aggregation issues etc.), so they have studied the supramolecular organization of these NPs and their interaction with blood plasma proteins in relation to their analgesic activity.
(A) Simplified formula of the LENK-SQ prodrug, three types of which were studied, differing in the chemical grouping (denoted as the "spacer") between Squalene and Leu-enkephalin.(
(B) Schematic diagram of the interaction between a spherical nanoparticle of LENK-SQ bioconjugates and the model blood protein BSA.
Researchers from the NIMBE (CEA/CNRS) designed a study to further dig into the supramolecular structures of the obtained nano-assemblies and to lead a very simplified nanoparticle-protein interaction assay using BSA (bovine serum albumin) as a model protein, albumin being the major protein component of the blood (Figure 1B).
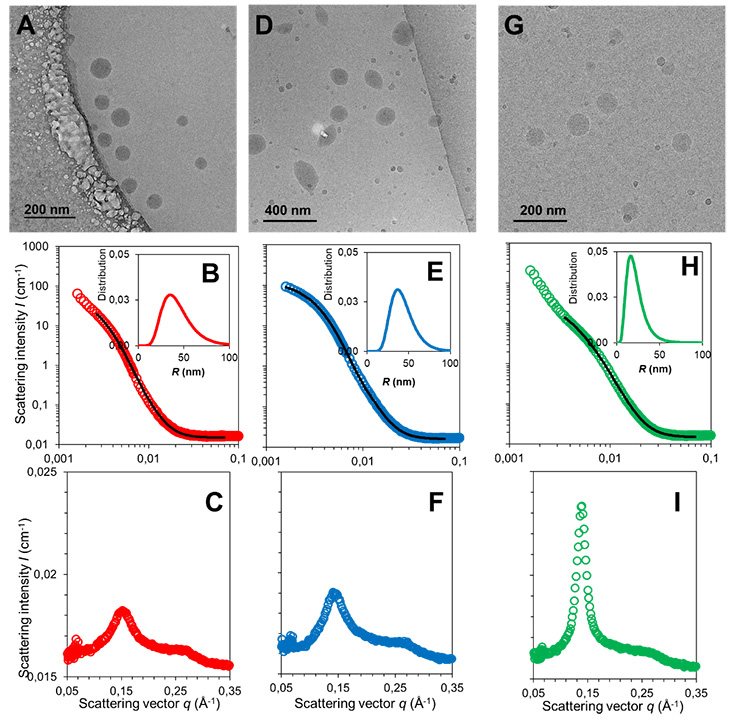
For this, they combined cryogenic transmission electron microscopy (cryoTEM), small- and wide-angle x-ray scattering (SAXS-WAXS), small angle neutron scattering (SANS) and synchrotron radiation circular dichroism (SRCD). Both SAXS-WAXS and SRCD analyses were performed at SOLEIL on respectively the SWING and DISCO beamlines.
Combined SAXS/WAXS and SANS are powerful techniques to analyse the size distribution and inner structure of nanoparticles for a large ensemble of organic particles (more than 1010). While SANS is traditionally preferred to study low concentrated organic suspensions in deuterated water taking benefit of the high sensitivity between the hydrogen of the nanoparticles and the deuterium from the solvent, SAXS/WAXS analyses provide useful indications on the NAs inner structure thanks to its better resolution.
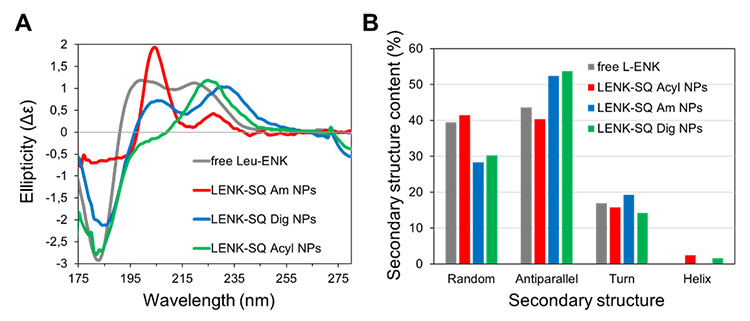
The results obtained show that a small change in the nature of the linkage between the peptide and the SQ moiety leads to different size distributions, charges, and different inner structures. The SRCD spectra show that while the overall drug conformation is retained in the NPs, in some cases it can be involved in the structure through participation in H-bonds.
(A) Synchrotron radiation circular dichroism spectra of the three NP suspensions along with the spectrum of free LENK in solution (2 mg/mL).
(B) Secondary structure analysis of the peptides.
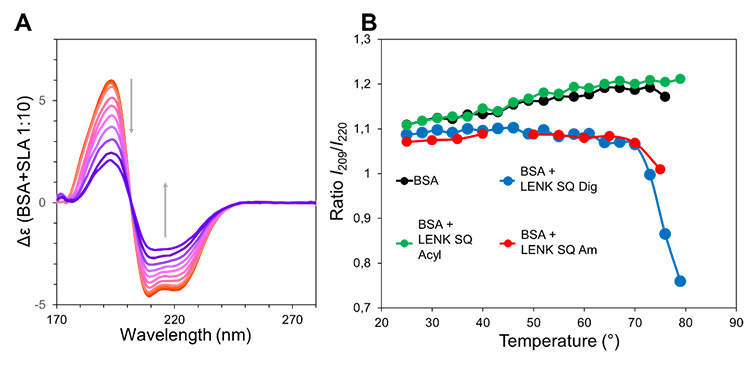
Concerning the interaction of the NPs with BSA, both SAXS and SANS show a decrease of the NPs scattering, suggesting that a disassembly mechanism is taking place, albeit with different magnitude depending on the nature of the linkage. The SRCD analyses of the BSA conformation in the presence of NPs confirm that a mild interaction exists between the protein and the prodrug, suggesting the formation of a complex.
(A) Circular dichroism monitoring of thermal denaturation of BSA in the presence of an excess of LENK-SQ Am NPs.
(B) Plot of the CD-intensity ratio at 209 and 220 nm during the thermal denaturation of BSA in solution and in the presence of LENK-SQ NPs Am, Dig, and Acyl, respectively.
As a conclusion, different physico-chemical features of the three parent prodrugs NPs were observed that could be put in relation to their different pharmacological activities previously observed. However, additional studies are needed to demonstrate a real causation.
This first study points towards mostly qualitative observations that will later be reinforced by quantitative analyses combined with theoretical studies to better describe the interactions between proteins and the prodrug NPs. Nonetheless, this study confirms that full physico-chemical analyses cannot be overlooked if we want to decipher the mechanisms of action of nanodrugs.
-----------------------------------------------------
* prodrug: a drug administered in an inactive form, which becomes active after transformation (by chemical reaction, usually by enzymes) in vivo. Here, the transformation is the release of the LENK moiety by cutting LENK-SQ at the spacer.