Experimental speciation of plutonium in seawater
The determination of the predominant forms of radionuclides in the marine environment has become a major area of research for many research institutes specializing in the impact of military and civilian nuclear activities on the environment. In this context, the Nice Chemistry Institute (ICN), CEA DAM DIF and CEA DEN DMRC in Marcoule have partnered with the MARS beamline at SOLEIL to determine the chemical form of plutonium in seawater. Understanding these chemical forms is absolutely essential to help model the transport mechanisms of plutonium at a very large scale—in cases of contamination accidents, for example.
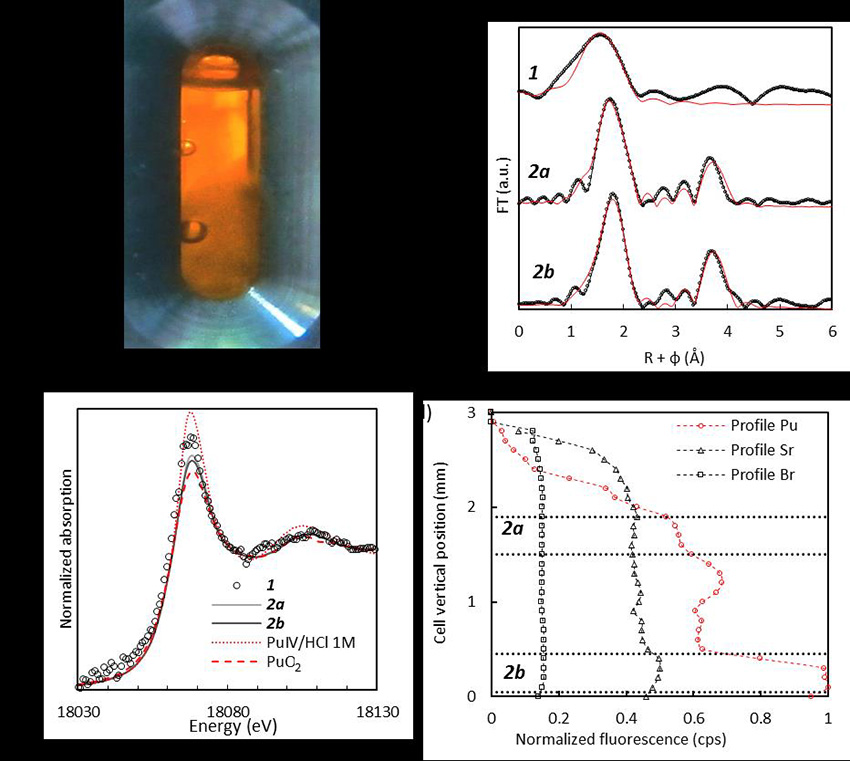
Samples representative of seawater (500 mL) collected off the coast of Monaco by a local team from IAEA (the International Atomic Energy Agency), were plutonium-doped to 10-5 mol.L-1 at CEA laboratories, then placed in a sample cell for the analysis of radioactive samples using X-ray absorption (Figure a). They were transferred to beamline MARS under the control of the radioprotection services at CEA and SOLEIL, and analyzed using X-ray absorption spectroscopy during a first measurement campaign. In parallel, the samples were analyzed using alpha-particle spectroscopy at the ATALANTE facility of CEA Marcoule. The successive series of measurements using alpha-particle spectroscopy indicate that the concentration of plutonium in solution decreases rapidly (< 10-6 mol.L-1 after 7 days) and lead, after several weeks, to the formation of a gel containing the majority of the plutonium introduced initially. The scientists carried out a second measurement campaign at SOLEIL to characterize the gel.
The XANES spectra (Figure b) recorded on the samples of doped seawater (first campaign) and on the gel (second campaign) reveal that the plutonium is predominantly tetravalent (Pu4+). Comparisons with reference data for the aquo ion [Pu(H2O)9]4+ and plutonium dioxide PuO2 indicate that the plutonium in seawater is quickly hydrolyzed in the initial doped solution, and that after a few weeks, it forms the gels described in the first campaign. Analysis of EXAFS spectra at 7 days (Figure c, spectrum 1) and 6 months (Figure c, spectra 2a and 2b), and the concentration profile (Figure d) obtained using X-ray fluorescence with the beam scanning vertically across the cell reveal a correlation between gel formation and slow plutonium(IV) hydrolysis. This phenomenon of polycondensation is of great interest as it could lead to plutonium immobilization mechanisms, while a soluble form, for instance, would be much more mobile.
Combined with conclusions from previous studies on uranium and americium, these results on plutonium allow for the first description of the predominant chemical forms of actinides in the marine environment. In a context where ongoing studies focus mainly on large-scale migration phenomena and few data is available at the molecular level, these results prove essential to feed impact prediction models.