The cyclic ground state structure of HF trimer revealed by the Jet-AILES device.
On the AILES beamline at SOLEIL, the supersonic jet device associated with high-resolution infrared (IR) absorption spectroscopy (Jet-AILES) brought definite proofs of the planarity of hydrogen fluoride cyclic trimer in its ground state.
This experimental challenge could not be addressed since the first studies realized at the end of the 80’s on that molecular complex. Finally a successful conclusion was obtained, thanks to the works of a French laboratory from the University Pierre et Marie Curie (MONARIS), the laboratory of Laser Physics, Atoms and Molecules in Lille, the Institute of Physics in Rennes, and the SOLEIL synchrotron. Scientists combined the broad spectral range attainable with interferometry and the high detectivity of this supersonic jet in the far IR. This spectral domain still little investigated turns out to be particularly interesting for the study of hydrogen bonding within small model complexes. Indeed, it allows the direct probe of newly formed intermolecular modes, avoiding the major constraints of vibrational dynamics at higher excitation energy
Small complexes formed from the bondings between two or three molecules (dimers, trimers) are the easiest systems to characterize binary and ternary intermolecular interactions which play an essential role to understand the condensed phase of matter. If we make a parallel with sociology, it clearly appears that interactions between a large number of molecules (or human beings) cannot be described as the sum over all possible pair interactions. The simplistic model called “pairwise additivity” is only an approximation and three-body intermolecular forces must be taken into account to describe more accurately molecular complexes beyond dimer. About the molecular trimers for example, this contribution represents only a small fraction of the total sum of forces and could only be extracted if binary interactions have been well modelled.
Among homo-molecular dimers (with the same molecule), hydrogen fluoride (HF)2 is one of the most studied systems for 30 years, especially using gas phase IR and microwave spectroscopy.
Very few spectroscopy studies have been reported on homo-molecular trimers up to now. Adiabatic relaxation (without any heat exchange) of a supersonic jet is the most adapted diluted environment for such systems. It enables to stabilize intermolecular bonds at low temperature, and then infrared spectroscopy is used to probe hydrogen bonded complexes in nearly isolated molecule conditions. Molecular parameters are deduced from the analysis of high resolution vibrational spectra resolved in rotation. In the case of the hydrogen fluoride trimer (HF)3, the high degree of symmetry of the cyclic structure prevents the use of some spectroscopic techniques
Jet AILES, efficient probe of the intermolecular forces in the far IR
In order to reveal the structure of such a trimer, scientists have implemented an experimental strategy aiming at exploiting a continuous high molar gas flow supersonic jet device in the far IR thanks to the high resolution spectrometer on AILES beamline. This experimental challenge is ambitious, because intermolecular modes of vibration are usually less intense and the detectivity of experiments lower than in the mid IR range. First, HF trimer signal was optimized in this more favorable spectral region, in order to establish experimental conditions for high resolution far IR experiments.
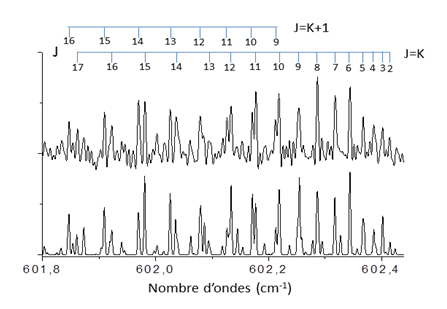
Rotationally resolved band contours of two bending modes, in-plane (v6) and out-of-plane (v4) of (HF)3 were observed in this region, and then globally analyzed to obtain the molecular parameters in ground and excited vibrational states. Figure 1 displays the comparison between the experimental spectrum and our best simulation of the rotational branch qQ(J,K) of the v4 mode.
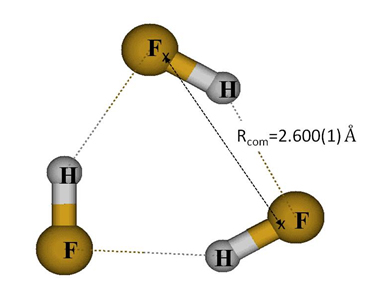
The question of (HF)3 planarity in the ground state could be definitely solved by exploiting information related to rotational parameters. More precisely, researchers confirmed that the (HF)3 planarity criterion for the ground state - represented with a simple relationship between centrifugal distortion constants, is fulfilled. Another strong argument is given by inertial defects1 deduced from rotational parameters: their values are well correlated with the expected geometry for the three vibrational states involved, close to zero in the ground state and for the in-plane bending mode, and much higher for the large amplitude out-of-plane motion. Lastly, the center of mass separations of the HF molecules (Rcom) have been precisely determined with an uncertainty of about one thousandth of angstrom (Figure 2).
In conclusion, scientists clearly proved through this study that the detectivity of the Jet-AILES device was sufficient enough to elucidate the structure of small molecular complexes, thanks to the successful association of broad-band high resolution IR spectroscopy and supersonic molecular jet technique. This experiment will be repeated soon, in order to characterize the structure of HF tetramer.
1Inertial defect: difference between moments of inertia along the principal axes of the molecular system studied. Its value is strictly zero for a planar molecule.