Phosphorus doping for higher-performance lithium-sulfur batteries
Metal borides have emerged as promising sulfur electrocatalysts for lithium-sulfur (Li-S) batteries. However, optimizing their electrocatalytic activity and understanding the underlying mechanism remain key challenges.
In a recent study, researchers from the TEMPO beamline and Nanjing University of Science and Technology (NJUST) reported a significant breakthrough by introducing phosphorus doping into nickel boride. This modification enables fast and durable sulfur electrochemistry, leading to a significant enhancement in Li-S battery performance.
As demand for electrical vehicles and grid-scale energy storage grows, conventional lithium-ion batteries (LIBs) face increasing difficulty meeting high energy performance and autonomy requirements. Li-S batteries offer a compelling alternative due to their exceptionally high theoretical energy density (2600 Wh kg-1), low cost, and environmental benignity. However, their practical implementation is hindered by the polysulfide shuttle effect1 and sluggish sulfur conversion kinetics. These issues become more severe under practical conditions, such as high sulfur loading and limited electrolyte dosage, resulting in low practical capacity and rapid battery failure.
Sulfur electrocatalysts can mitigate these challenges by promoting sulfur redox reactions. Among the various candidates, metal borides (MBs) are gaining attention due to their excellent metallicity and conductivity, which surpass most other metal compounds. Moreover, their local metal-boron ionicity provides abundant active sites for polysulfide adsorption and catalytic conversions. Despite these advantages, MB-based sulfur electrocatalysts remain underexplored. Traditional MB preparations often require harsh conditions, such as high temperature and pressure, and precise structure tuning to maximize catalytic efficiency is still lacking.
To address this, the team employed a heteroatom-doping strategy, introducing phosphorus into nickel boride to enhance the catalytic activity and ultimately achieve significant improvement in Li-S battery performance.
Results
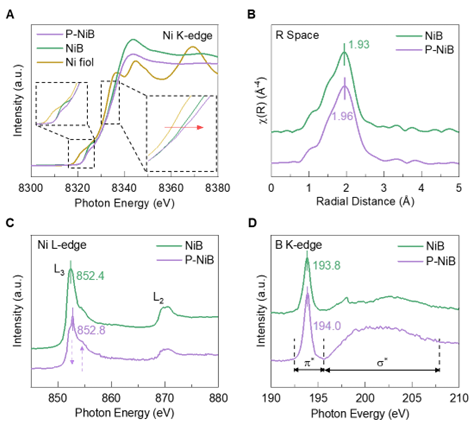
The phosphorus-doped nickel boride (P-NiB) was prepared via a facile and mild solution-based method. Spectroscopic analysis via XANES and EXAFS revealed intensified local disorder and lattice asymmetry in P-NiB, along with a reduced B 2p orbital occupancy compared with its undoped counterpart (NiB) due to the electron transfer from Ni/B to P. This electronic modulation significantly strengthens B-S p-p interactions, leading to enhanced polysulfide fixation and accelerated bidirectional sulfur conversions.
The kinetic advancements were confirmed by the symmetric cell and potentiostatic Li2S redox texts, where P-NiB showed a significantly higher current response and reduced overpotentials for sulfur redox conversions compared with NiB. In addition, CV profiles, operando XRD, and in-situ Electrochemical Impedance spectra also demonstrated reduced activation energy and promoted sulfur reactions in coin cells based on P-NiB, collectively confirming the significantly enhanced catalytic activity after P-doping.
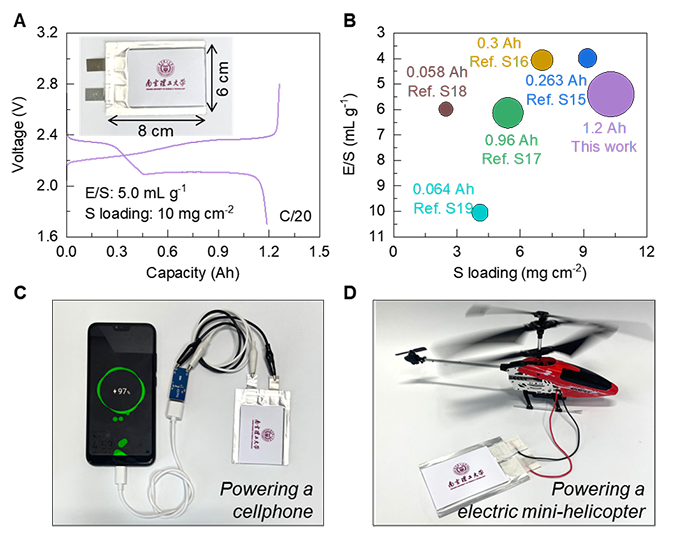
As a result, Li-S cells incorporating P-NiB delivered excellent performance in terms of capacity, rate capability, and cycling stability, even under demanding conditions such as high sulfur loading and low electrolyte-to-sulfur (E/S) ratio. A 1.2 Ah multilayer pouch cell based on this catalyst outperformed most recent benchmarks and was capable of powering a cellphone and a mini-helicopter, showcasing its good potential for practical applications.
Conclusion
Phosphorus doping in nickel boride effectively tailors its electronic structure by lowering B 2p orbital occupancy, thereby enhancing B-S p-p orbital interactions. This facilitates stronger polysulfide adsorption and faster redox kinetics. The study provides compelling evidence of how anion engineering can optimize the electrocatalytic properties of metal borides, advancing their application as high-performance sulfur electrocatalysts for next-generation Li-S batteries.
1 - shuttle-effect: In Li-S batteries, lithium ions move between the cathode and anode by a chemical reaction. Elemental sulfur from the cathode is converted into polysulfide compounds — composed of sulfur atom chains — some of which can dissolve in the electrolyte. Because of this solubility, a “shuttling” effect occurs, where the polysulfides travel back and forth between the cathode and the anode, resulting in loss of material from the sulfur cathode because it is deposited at the anode. This effect limits the overall battery cycle life and performance.