➋
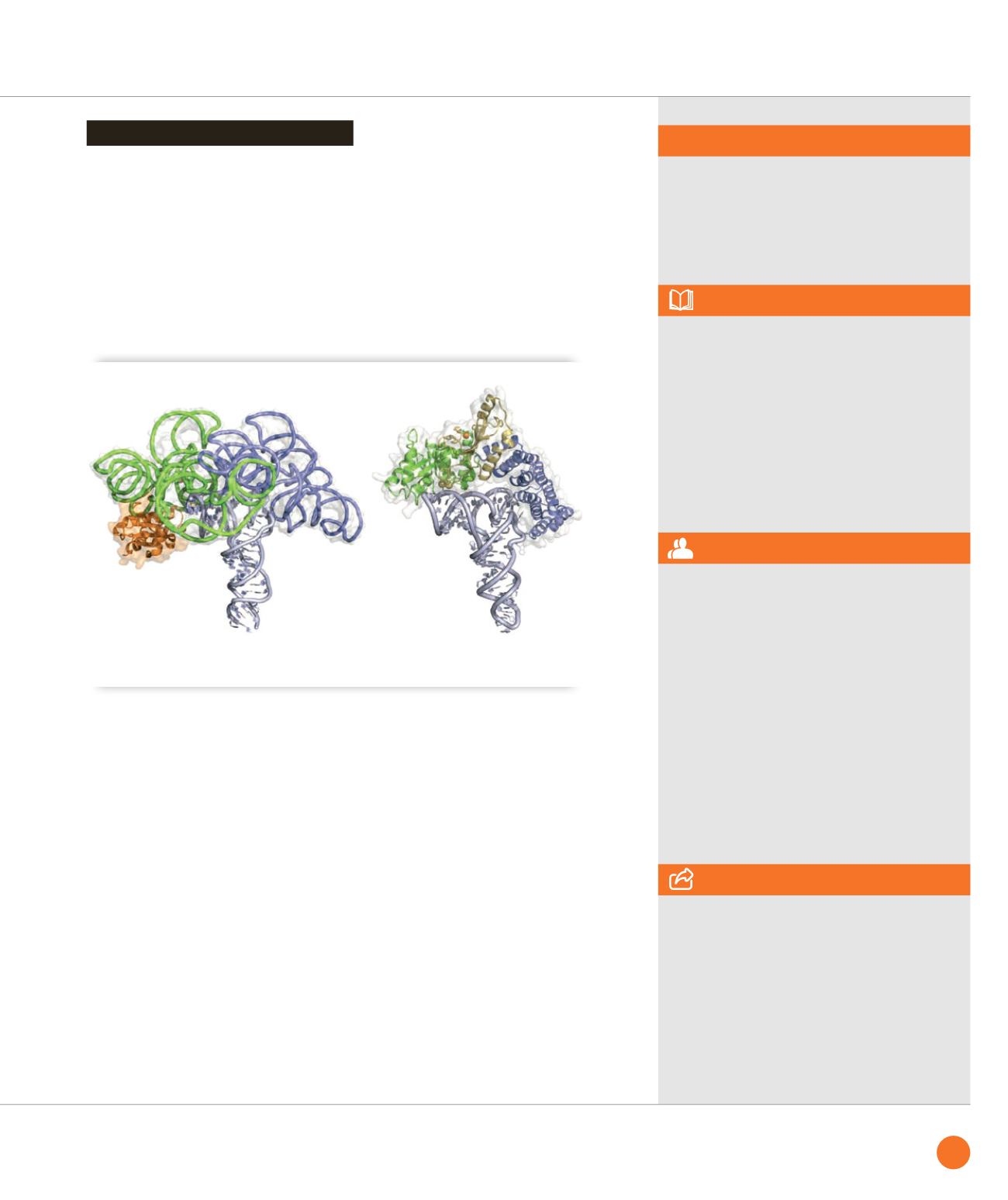
Classical ribonucleoproteic RNase P (left, PDB id: 3Q1R) and PRORP2 (right, model based on PDB id: 4G26)
share the same pre-tRNA binding mode [4].
SWING & DISCO beamlines
ASSOCIATED PUBLICATION
Structural insights into protein-only RNase P
complexed with tRNA
A. Gobert, F. Pinker, O. Fuchsbauer,
B. Gutmann, R. Boutin, P. Roblin, C. Sauter*
and P. Giegé.
Nature Communications 4 (2013) art.1353
REFERENCES
[1] Holzmann et al. Cell 135 (2008), 462
[2] Gobert et al. Nat. Struct. Mol. Biol.
17 (2010), 740
[3] Gutmann et al. Genes Dev. 26 (2012), 1022
[4] Pinker et al. RNA biology 10 (2013), 1457
*Laboratoire “Architecture et Réactivité
de l'ARN”, UPR 9002 du CNRS, Institut
de Biologie Moléculaire et Cellulaire (IBMC),
Université de Strasbourg,
15 rue René Descartes, 67084 Strasbourg,
France
CORRESPONDING AUTHOR
To position PRORP on its RNA substrate,
the latter was subjected to RNase digestion
in the presence of the enzyme. The
protection footprint (Figure
➊
) defined
the interaction interface and the PRORP
enzyme was docked accordingly onto the
3D structure of a pre-tRNA. This model
of the maturation complex reveals that
eukaryotes have evolved PPR proteins to
recognize pre-tRNAs in a similar way as
the ribonucleoproteic RNase P reminiscent
from the ancient
RNA world
(Figure
➋
).
Although the scenario of this convergent
evolution remains to be established,
as well as the precise catalytic mechanism
of tRNA maturation, this study is a first step
towards the detailed characterization of the
PRORP family.
Probing PRORP:tRNA interface
65
SYNCHROTRON
HIGHLIGHTS
2013


