The novel RNase P
in action
A novel type of RNase P was recently
identified which is totally deprived of
catalytic RNA. This proteinaceous RNase P
(or PRORP) is found in the organelles
of many eukaryotes and in the nucleus
of some eukaryotes including plants.
In order to characterize the architecture
of PRORP enzymes and to determine how
they bind to pre-tRNAs to perform their 5'
maturation we combined biochemical
and biophysical approaches. The resulting
model of a functional PRORP:substrate
complex suggests a tRNA recognition
mode similar to that of the ribonucleoproteic
RNase P.
Transfer RNAs (tRNAs) are key actors
of protein synthesis: they play the
role of adapter molecules during the
translation of messenger RNAs by the
ribosome into protein sequences. They
are produced as precursors with leading
and trailing sequences that need to
be processed. Their 5’ maturation is
catalyzed by a ubiquitous enzyme called
RNase P. Until recently all known RNase
P were ribonucleoproteins, the catalytic
activity of the enzyme being held by an
RNA molecule. In 2008, a new type of
RNase P only composed of proteins was
identified in human mitochondria [1] that
corresponds to a novel family of nucleases
called PRORP for “Proteinaceous RNase
P”. The group of Philippe Giegé (Institut
de Biologie Moléculaire des Plantes, IBMP,
Strasbourg) demonstrated that the model
plant
Arabidopsis thaliana
possesses
three PRORP proteins. PRORP1 is localised
in both mitochondria and chloroplasts
whereas PRORP2 and PRORP3 are active
in the nucleus [2,3]. A collaboration was
initiated between two neighbouring
institutes in Strasbourg (IBMP and IBMC)
to examine these enzymes from
A
.
thaliana
and a combination of biochemical and
biophysical approaches was used to gain
a first structural and functional insight
into tRNA recognition and maturation
by PRORPs.
5' tRNA maturation in mitochondria
BIOLOGY AND HEALTH SCIENCES
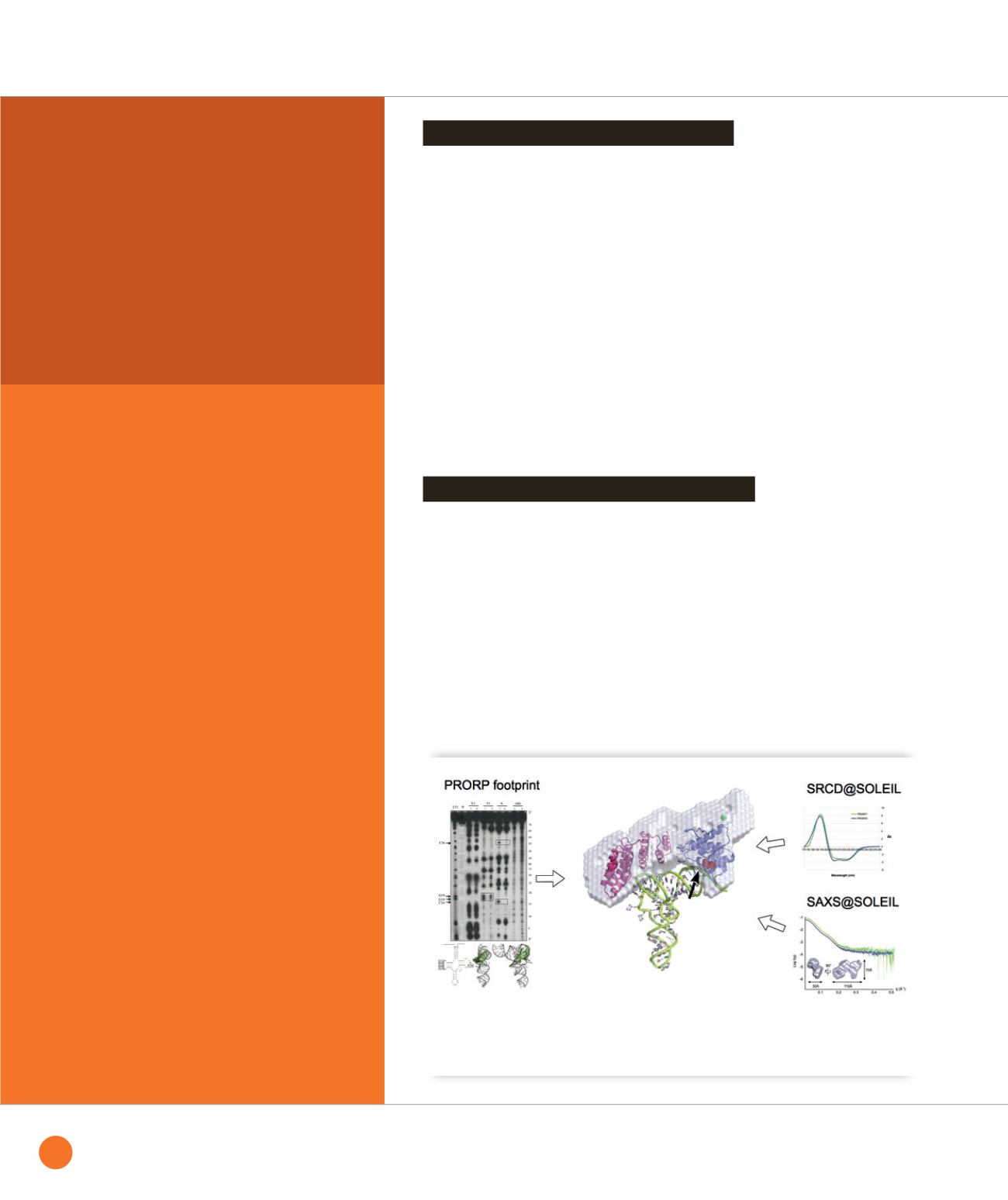
➊
A first glance at a PRORP:tRNA complex. PRORP2 of
A
.
thaliana
was built by comparative modelling guided by
SRCD and SAXS data, and the resulting model was docked onto a pre-tRNA substrate based on footprint analysis.
The RNA cleavage position is indicated by an arrow.
PRORP sequences are characterized by
the presence of pentatricopeptide repeat
(PPR) motifs and a metallonuclease
domain proposed to hold the catalytic
center. Because no structure of a close
homologue was known at the time we
started this study, comparative modeling
was carried out on separate domains.
We then performed synchrotron radiation
circular dichroism (SRCD) on the DISCO
beamline to validate the models based
on their 2D structure content and to test
the conformational stability of PRORP
samples prior to further investigations. The
presence of a zinc binding motif between
the two main domains was demonstrated
by site directed mutagenesis of putative
zinc chelating residues in association
with inductively coupled plasma mass
spectrometry. Small angle X-ray scattering
(SAXS) data collected on the SWING
beamline confirmed the two domain
organization of PRORPs and helped place
them with respect to each other (Figure
➊
).
PRORP: an integrated structural study
64
SYNCHROTRON
HIGHLIGHTS
2013


