➊
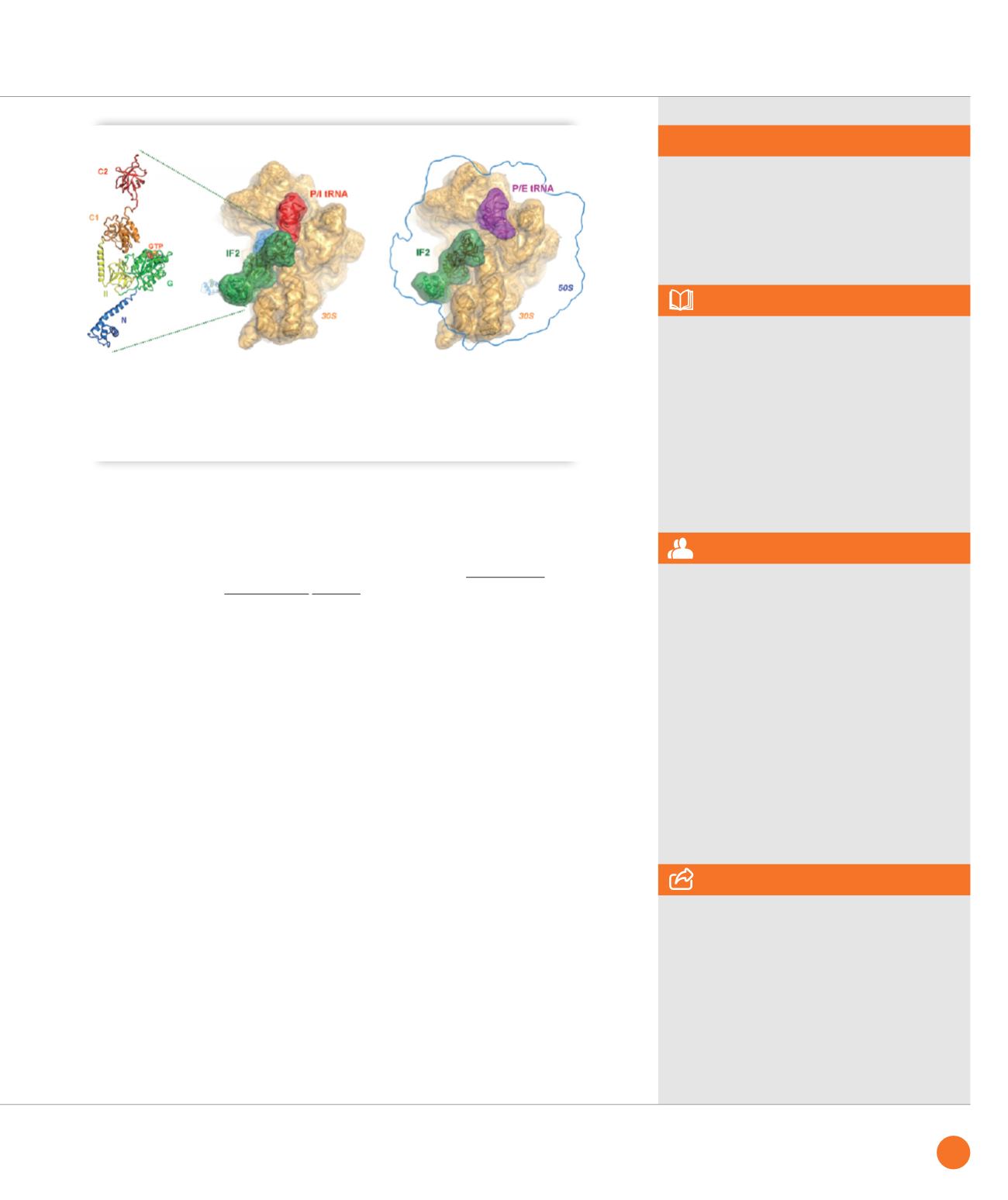
Structure of the multi-domain translation initiation factor IF2 as seen in isolated form (left, crystallography and
SAXS) and when bound to the 30S subunit of the ribosome (middle, cryo-EM) where it stabilizes the initiator tRNA
(labelled in red). When the N-terminal domain (marked in blue in the left panel) is removed, 70S formation stalls
in a non-productive state (right panel, cryo-EM) with the initiator tRNA (magenta) in a P/E-site transient position
and subunit joining is affected as monitored by fast kinetics and single molecule fluorescence analysis.
SWING beamlines
ASSOCIATED PUBLICATION
Involvement of protein IF2 N domain
in ribosomal subunit joining revealed from
architecture and function of the full-length
initiation factor
A. Simonetti, S. Marzi, I.M. Billas, A. Tsai,
A. Fabbretti, A.G. Myasnikov, P. Roblin,
A.C. Vaiana, I. Hazemann, D. Eiler, T.A. Steitz,
J.D. Puglisi, C.O. Gualerzi and B.P. Klaholz*
Proc Natl Acad Sci U S A, 110(39) (2013) 15656
REFERENCES
[1] A. Simonetti et al. Nature
455 (2008), 416
[2] A. Simonetti et al. Acta Cryst.
D69 (2013), 925
[3] D. Eiler et al. Proc Natl Acad Sci U S A
110 (2013),15662
* Centre for Integrative Biology, IGBMC,
67404 Illkirch, France
CORRESPONDING AUTHOR
The multi-approach technique showcased in this study was possible by the access
to the SOLEIL synchrotron facilities and to the FRISBI infrastructure
and its Strasbourg node Instruct France Centre 1 which is also available to all Instruct
member countries in Europe.
67
SYNCHROTRON
HIGHLIGHTS
2013


