Polymorphic and amyloid
natures of neuronal
inclusions of Huntington’s
disease brain revealed
by IR microspectroscopy
Huntington’s disease (HD) is a
neurodegenerative disease characterized
by the formation of protein aggregates
in certain regions of the brain; some
of these aggregates can reach microscopic
size (inclusions). Studies in cells and
animals have shown that aggregates
are polymorphic and that their secondary
structure is likely to condition their
toxicity [1]. However, the secondary
structure of proteins in the inclusions
found in the brain of patients
is still unknown. We show by using
synchrotron Fourier-transform infrared
microspectroscopy (sFTIR), that the brain
of HD patients contains structurally different
inclusions, some of which are amyloid.
As one category of amyloid inclusions
is characteristic of severely affected
brain regions, it may be particularly
toxic to neurons.
HD results from a mutation in a gene
encoding a protein called huntingtin [2].
Huntingtin contains a chain of 20-35
consecutive glutamines in healthy
individuals, whereas this chain is
expanded from 36 to 65 glutamines in
patients with the adult form of HD and
exceeds 65 residues in those with the
much rarer and more severe juvenile form.
The disease is mainly characterized by
a progressive destruction of the striatum
and also to a lesser extent of the cortex [3],
and by the formation, in these regions,
of inclusions [4] (Fig.
➊
). Inclusions are
located mostly in the cell cytoplasm (Cis)
in adult cases and in the cell nuclei (Nis)
in juvenile cases. Thanks to the high-
sensitivity and the high-resolution
provided by the synchrotron source,
we have analyzed by sFTIR the structural
nature of inclusions
in situ
in post-mortem
brain of patients affected by HD. We
have investigated the protein secondary
structure of Cis and Nis present in the
cortex and the striatum of adult as well
as of juvenile HD cases.
We acquired IR spectra from Cis and from
inclusion-free cytoplasm (as controls) of
five adult HD cases. Differences between
spectra were then studied by average
second derivative spectra and principal
component analysis. We found that
cortical and striatal Cis display increased
contributions of peaks at 1627, 1681
and 1693 cm
-1
, all indicating
β
-sheet
enrichment (Fig.
➋
). The 1627 cm
-1
peak
is also a signature of the amyloid nature
of these inclusions [3]. We showed that
striatal Cis differ from cortical Cis by the
presence of an additional component
at 1639 cm
-1
, assigned to “
β
-sheet/
unordered” structures.
We photographed areas encompassing
fluorescently labeled Cis and generated
chemical maps representing the
β
-sheet/
β
-helix ratio. The immunolabeled area and
the area with the highest
β
-sheet/
α
-helix
ratio were clearly superimposable (Fig.
➌
).
We discovered that Cis in juvenile HD
cases did not differ appreciably from
the surrounding cytoplasm, whether
in the cortex or the striatum (Fig.
➋
).
Therefore, they constitute amorphous,
non-amyloid protein aggregates.
Introduction
Cytoplasmic inclusions in adult HD cases
have different amyloid structures in cortex and striatum
Cytoplasmic inclusions in juvenile HD cases
constitute amorphous aggregates
BIOLOGY AND HEALTH SCIENCES
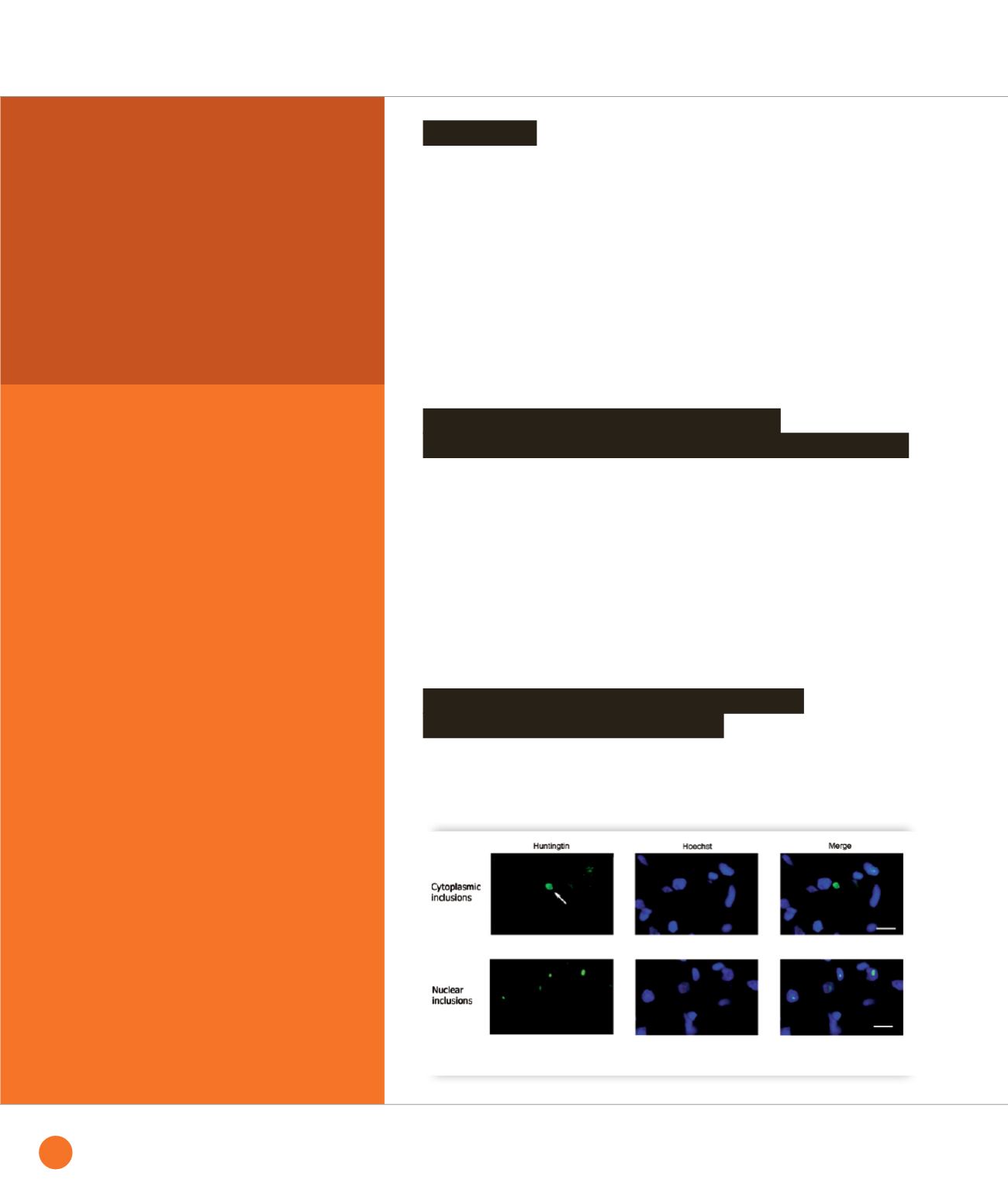
➊
Images of fluorescently labeled inclusions in HD brain showing one cytoplasmic inclusion (upper panels)
and numerous smaller nuclear inclusions (lower panels).
54
SYNCHROTRON
HIGHLIGHTS
2013


