The transfer of TiO2 nanoparticles in the food chain depends on soil texture
Researchers from the Laboratory for Functional Ecology and Environment (EcoLab) in Toulouse, Grenoble CEA (INAC), the nuclear microprobe in Saclay CEA (LEEL) and the LUCIA beamline at SOLEIL have studied the influence of the nature of the soil on the outcome of nanoparticles, more and more present in our environment.
For a little more than a decade, nanotechnologies have attracted the interest of industrials and scientists throughout the world. The number of commercial goods available on the market and (officially) containing nanoparticles has increased from 54 in 2005 to 2850 in 2016 in all fields of everyday life. Among those nanoparticles, TiO2 nanoparticles are one of the most used, appearing in about 25% of the products according to nano-databases. This expansion of TiO2 nanoparticle use is inevitably leading to an increased dissemination in the environment. Moreover, the spreading of sewage sludge, which is concentrated in nanoparticles (2 g.kg-1), on agricultural soils and the use of nanopesticides will locally increase nanoparticle concentration making agricultural soil the main sink for nanomaterials in the environment and crop plants a privileged point of entry for nanoparticles in the food chain up to humans.
Once nanoparticles reach the soil, they can be either physically retained or chemically adsorbed onto the surface of soil particle. These interactions could mitigate their phytotoxicity and bioavailability, meaning that a same contamination could lead to very distinct consequences according to soil type.
Thus for a better risk assessment a multidisciplinary team assembled to understand soil influence on TiO2 nanoparticle fate in an agroecosystem. This team was composed of researchers from the Laboratory for Functional Ecology and Environment (EcoLab) in Toulouse, from Grenoble CEA (INAC), from the nuclear microprobe in Saclay CEA (LEEL) and from the LUCIA beamline at SOLEIL.
Wheat, TiO2, sands and clays
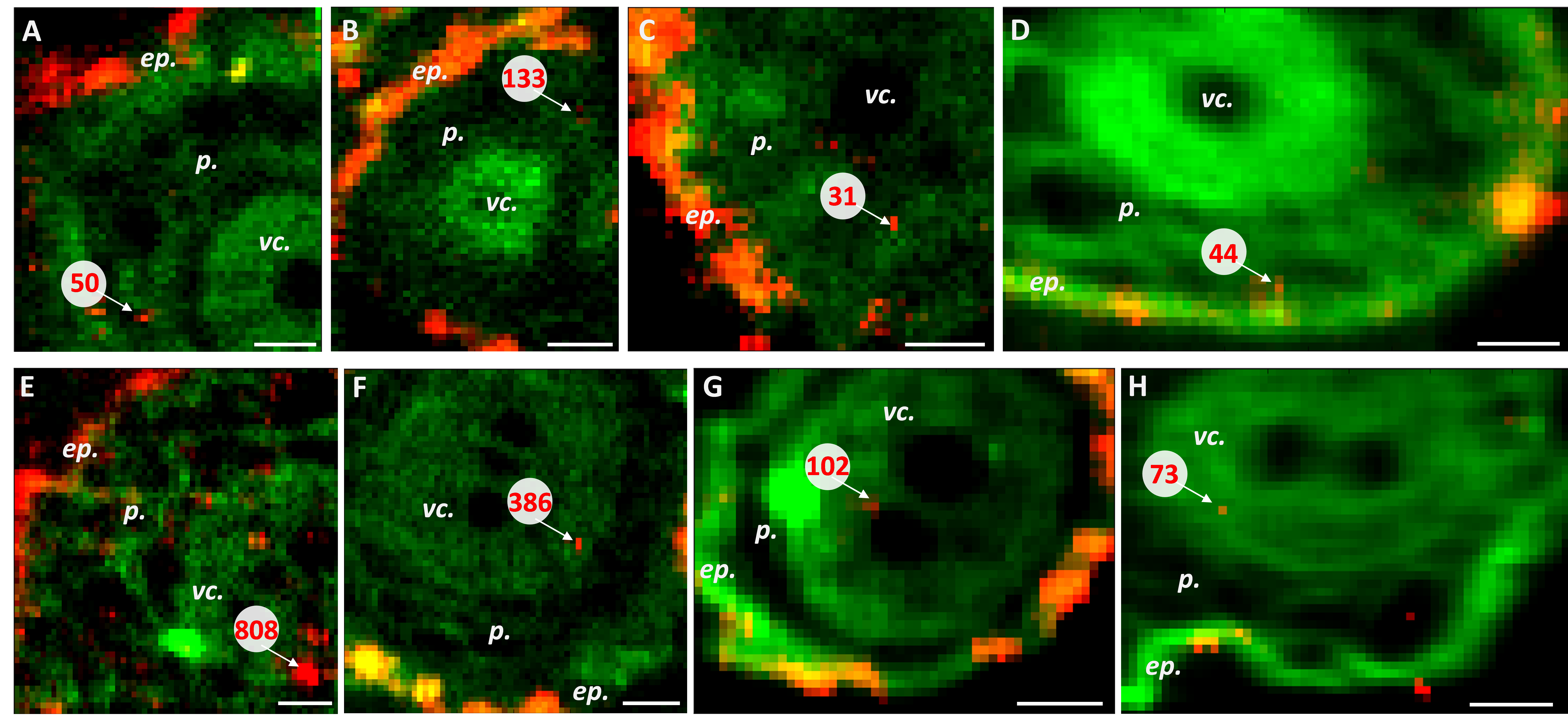
To understand soil influence, wheat, a widely grown crop over the world, was exposed to TiO2 nanoparticles (0, 100, 500 mg·kg−1) in 4 different types of soil. After several weeks of exposure, Ti quantification in soil, soil leachates1 and wheat leaves was assessed. Ti distribution in roots and leaves and speciation (is Ti complexed with other molecules, for example) in leaves were also determined using techniques available on LUCIA ( X-ray fluorescence microspectrocopy and micro-XANES) and at LEEL (micro particle induced X-ray emission, µPIXE, and Rutherford Backscattering spectroscopy, RBS, especially suitable for the study of light elements, C, N, O).
Finally nanoparticle phytotoxicity was evaluated through plant development parameters: height, fresh and dry weights and chlorophyll content.
The results obtained in this study demonstrated that TiO2 nanoparticles can be significantly taken up with no speciation modification by wheat seedlings after a 3 week exposure in a sandy soil in which 500 mg·kg−1 of engineered nanoparticles have been added (Figure 1). This is also in this soil type that nanoparticles were able to reach in higher quantities the leachates. Thus if a contamination occurs in a sand, nanoparticles will be very mobile towards terrains storing water and bioavailable to crop plants representing a potential risk for trophic transfer. However, sand is not a relevant medium for agriculture. The same trend has been highlighted in silty sand but in smaller proportions. In contrast, in loamy sand and clayey loam no difference has been evidenced after addition of nanoparticles to the system: no Ti concentration increase in the soil, partly due to the high natural Ti concentration in the soil, no increase in plant roots and leaves and little to no increase in the soil leachates. This difference in TiO2 nanoparticle behavior can be related to 3 characteristics of the soil used: organic carbon content, clay content and cationic exchange capacity. In all conditions, no acute sign of phytotoxicity was detected in the endpoints analyzed.
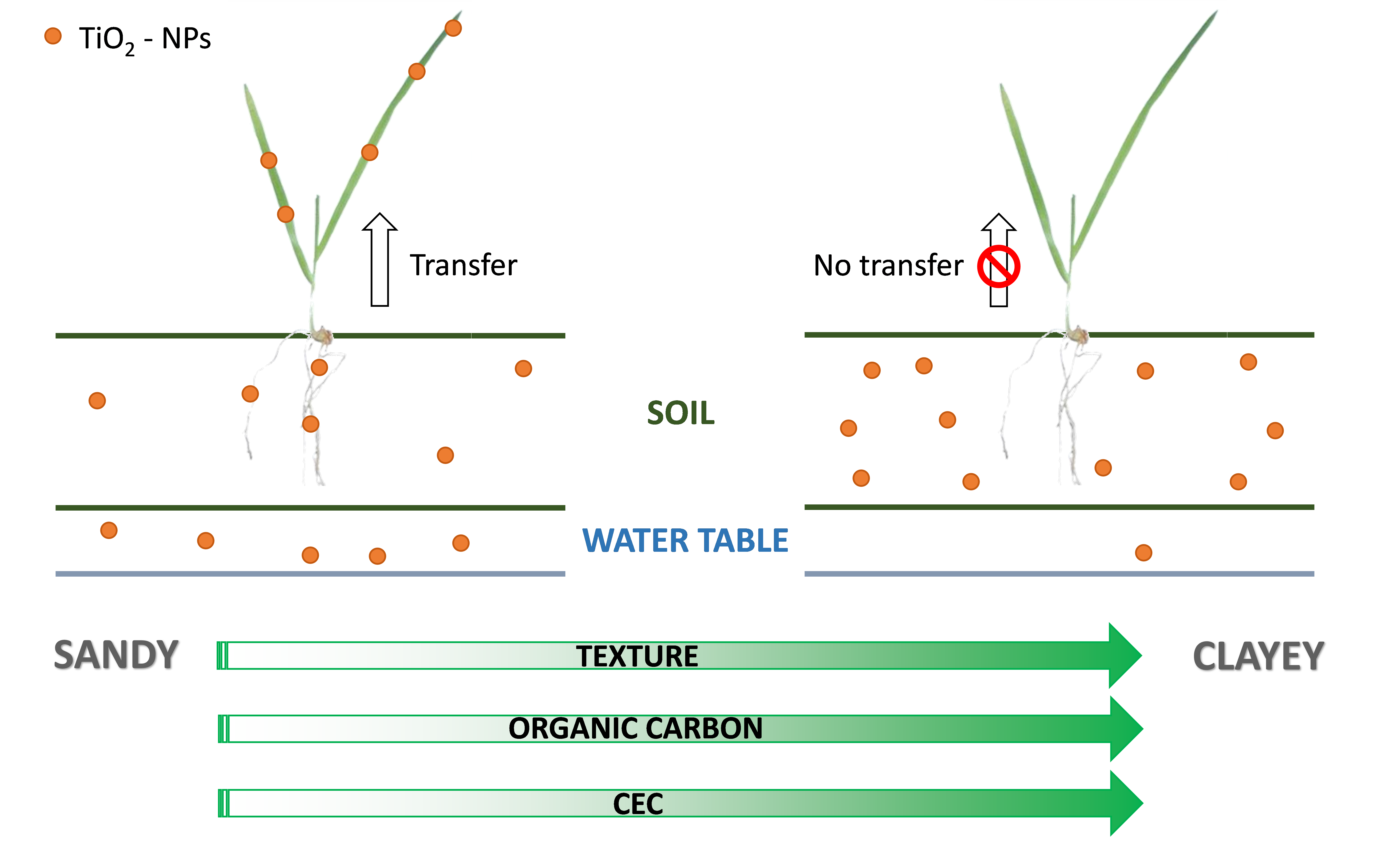
The overall conclusion for agro-ecosystems is that TiO2 nanoparticle contamination (after a short term exposure: 3 weeks) seems to have little impact on plant health and low chance of uptake as well as low risk of leaching to the aquifers (Figure 2). The soil displaying the highest risk for food safety would be the silty sand but this soil had little nutrients for plant growth and was not the best featured for agriculture.
However, those results also imply that TiO2 nanoparticles will remain in the soil with possible consequences for soil micro and macro-organisms.
1 – leachate: in order to contaminate the soil it is mixed with a suspension of nanoparticles, then the excess of liquid (leachate) percolates through the soil.