Tracking nano heat damages in tumor cells
By combining nanoscale thermometry with cell-scale imaging, researchers have revealed how heat generated by gold nanostars under near-infrared light travels from individual nanoparticles to entire tumor cell structures.
Using 3D tumor models and X-ray absorption spectroscopy at SOLEIL’s SAMBA beamline, scientists measured atomic-level temperature changes and observed how these trigger the cellular damages.
When gold nanostars (AuNS) absorb near-infrared light, they convert it into heat capable of destroying cancer cells. The highest temperatures occur in the immediate vicinity of each nanoparticle, but these local values have been difficult to measure within biological tissue, as well as to link directly to thermal damage at the cell level.
An international team led by Institute of Materials Science of Madrid ICMM-CSIC (Spain), Complutense University of Madrid (Spain), Institut Curie (France), IMDEA Nanociencia (Spain), Institute of Ceramics and Glass ICV-CSIC (Spain), Valladolid University (Spain) and SOLEIL Synchrotron (France) has now combined nanoscale temperature sensing with high-resolution microscopy to follow heat effects across multiple scales, from Au atoms to entire tumour-like cell structures.
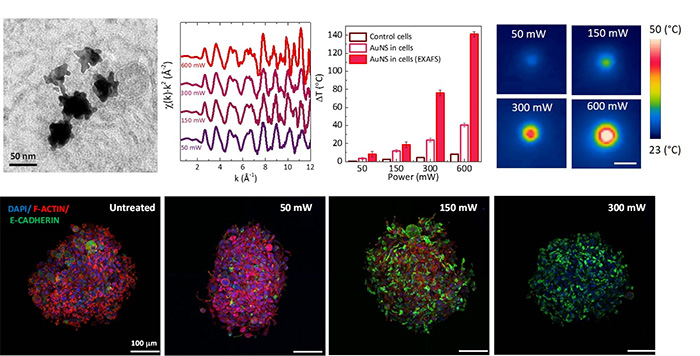
At SOLEIL’s SAMBA beamline, the researchers used extended X-ray absorption fine structure (EXAFS) spectroscopy to monitor atomic vibrations in AuNS embedded in living 3D tumour spheroids1. By calibrating these vibrations, quantified by the Debye-Waller factor, against known temperatures, they developed a precise “nanoscale thermometer.”
When AuNS-loaded spheroids were illuminated with an 808 nm laser, EXAFS data revealed that local nanoscale temperatures were higher than the values recorded by infrared cameras. This confirmed the presence of steep thermal gradients in the cellular environment that cannot be detected by standard bulk measurements.
To determine how these nanoscale heat spikes affect cells, the same spheroids were examined by confocal microscopy. At moderate heating, the cell–cell adhesion protein E-cadherin remained intact or was even reinforced, potentially stabilising tissue structure. At higher heating levels, the cell cytoskeleton (F-actin) collapsed. Above certain temperature thresholds, spheroids lost viability and disintegrated, a clear macroscale consequence of nanoscale thermal damage.
By correlating nanoscale temperature rises with microscale structural changes and macroscale tissue responses, this study delivers the first complete multiscale picture of photothermal therapy. The results open the way to optimising treatment conditions to maximise cancer cell destruction while preserving healthy tissue. Future work will focus on extending this approach to other nanomaterials and biological models, reinforcing the role of synchrotron X-ray techniques in bridging nanophysics and cell biology.
1 - A spheroid is an in vitro 3D tumor model of glioblastoma, it’s a spherical self-assembled aggregate of glioblastoma cells.