Selenium adsorption on coinage metals: chemisorption, polymerization and selenide films
Selenides e.g. bulk silver selenide are of much interest because of their use in various applications such as a thermoelectric materials, a photosensitizer in photography, in photochargeable batteries. Adsorption of sulphur on various metal surfaces has been investigated in great detail, but there are very few surface science studies of the characteristics of heavier chalcogenide* adsorption. This motivated the study by the ISMO (Juanjuan Jia and V.A. Esaulov) group’s study of selenium adsorption on Au(111) and Ag(111) surfaces. The study was carried out in collaboration with the group of Prof S. Sampath (Indian Institute of Science, IISc, India) for the electrochemical investigations, and the group at the TEMPO beamline (Azzedine Bendounan and Fausto Sirotti) for the photoemission experiments.
Recently it has been found that non-stoicheometric silver selenide displays giant magneto-resistance. These features have stimulated many recent studies of Ag2Se films, nanocrystals, and nanotubes. In recent years selenium1 and tellurium derived self-assembled monolayers (SAMs) have attracted much interest as alternatives to thiol-derived SAMs, in e.g. molecular electronics, because of different (Se as compared to S) bonding to the substrate: this is a point of key importance for electronic transport.
Adsorption of sulphur on various metal surfaces has been investigated in great detail, but there are still pending questions concerning e.g. the sulphur metal interaction in SAMs. On the other hand there are very few surface science studies of the characteristics of heavier chalcogenide* adsorption. This motivated the study carried out by scientists from ISMO, IISc and TEMPO, recently published in Journal of Physical Chemistry.
Se adsorption on Au and Ag
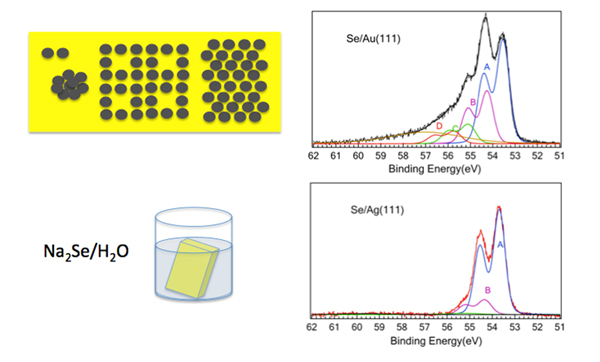
The Se adsorption was performed in a facile manner by dipping the pristine single crystal samples into a Na2Se solution in a NaOH aquous solution for a short time. This was performed in the SOLEIL chemistry laboratory by Karine Chaouchi, in an inert atmosphere glove box. After rinsing the sample, Se adsorption is indeed confirmed by X ray photoemission spectroscopy (XPS) with no remaining Na.
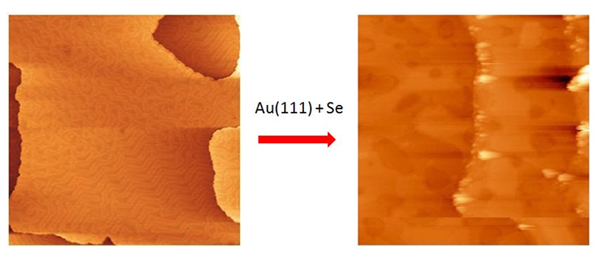
The cyclic voltammetry study of adsorption on gold at the Indian Institute of Science showed formation of selenium adsorbed species and structures observed in reductive desorption that were related to the atomic and polymeric2 species. The detailed high resolution XPS study in the case of the Au surface in the Se(3d) region in fact shows two main features (figure 1.), that by analogy to what is known for sulphur3 were attributed to Se chemisorbed atomically and to polymeric Se8 species. Some smaller features due to other species are also observed, which might be due to other Se conformations like e.g. dimer and bulk like Se. The deconvolution of the Au(4f) XPS spectra also shows that we are not dealing with a gold selenide film, but a chemisorbed Se layer, which does not lead to noticeable core level shifts of surface Au atoms. Some preliminary investigations by Scanning Tunneling Microscopy, performed in the Surface laboratory of SOLEIL in collaboration with Stefan Kubsky and François Nicola, indicated the formation of a very “patchy” surface, presumably due to the polymeric selenide overlayer. Both the XPS and electrochemistry study show that the polymeric component increases with incubation time.
Differences between gold and silver…
Notable differences in selenium adsorption on gold and on silver were revealed, starting from a noticeable darkening of the Ag surface as in photo toning. Indeed in case of adsorption on Ag(111) the XPS spectra for the Ag(3d) show a broadening of the peak to lower binding energies, that could be deconvoluted into AgB bulk like and a lower binding energy AgSe components, showing appearance of a surface Ag selenide. The shift to lower energies may appear somewhat strange from the point of view of the usual considerations based on the electrostatic potential model, but is similar to the shifts observed for Ag oxidation4. The Se(3d) XPS spectrum is found to be substantially different from the Au case and is dominated by atomic Se corresponding to the selenide, though a smaller intensity, as yet unidentified Se structure at an energy similar to the polymeric Se8 structure for Au, was also observed.
This study thus revealed rather different behaviours in Se interaction with the coinage metals Au and Ag. Adsorption on Au results in chemisorbed and polymeric Se formation, while on Ag a surface selenide film is formed. These precise high resolution XPS measurements will help to better understand the characteristics of both Se based self-assembled films and also Ag selenide nanostructures.
* chalcogenide: anion of the chalcogen group, i.e. oxygen, sulfur, selenium, tellurium, polonium.
References
1. M. Canepa et al. Phys Chem, Chem. Phys , 2013,15, 11559
2. T.E. Lister & J.L. Stickney, J. Phys. Chem. 1996, 100, 19568
3. P. G. Lustemberg et al. J. Phys. Chem. C, 2008, 112 (30), 11394
4. H. Gronbeck et al. Physical Review B, 2012, 85, 115445