Restoration and conservation of marine ferrous archaeological artefacts
The conservation of metallic artefacts coming from archaeological excavations requires stabilisation methods, such as dechlorination treatments, traditionally carried out in conservation workshops.
The dechlorination mechanisms of ferrous archaeological artefacts have been highlighted by direct in situ investigation of the transformation of the corrosion product layers, using X-ray diffraction on the DIFFABS beamline.
The alteration of artefacts in a marine environment
The physico-chemical study of iron ingots recovered during the archaeological excavations of a Gallo-Roman shipwreck (1st century BC) off the Saintes-Maries-de-la-Mer coast revealed corrosion products containing chlorine: akaganeite β-FeO1-x(OH)1+xClx and β-Fe2(OH)3Cl, a particularly unstable phase in an oxidizing medium. Certainly, the phases formed during the iron corrosion processes in various environments (marine, terrestrial or atmospheric) can fix certain salts from the medium, including chlorides. After excavation, and if not rapidly stabilized, the artefacts are commonly subjected to new corrosion processes, notably due to the presence of oxygen in the air. This often gives rise to rapid and unavoidable degradation of the corrosion product layers by cracking, causing the loss of unique and irreplaceable centuries-old objects.
Stabilisation by dechlorination
Dechlorination treatments are traditionally used in conservation workshops in order to stabilise objects from archaeological excavations (notably marine). The evaluation of the effectiveness of these treatments, their optimisation or the development of new protocols are part of the daily concerns of the professionals in charge of the protection of these heritage artefacts. Exhaustive knowledge of the transformation experienced by these artefacts during a dechlorination treatment could provide a better understanding to respond to these concerns with greater accuracy.
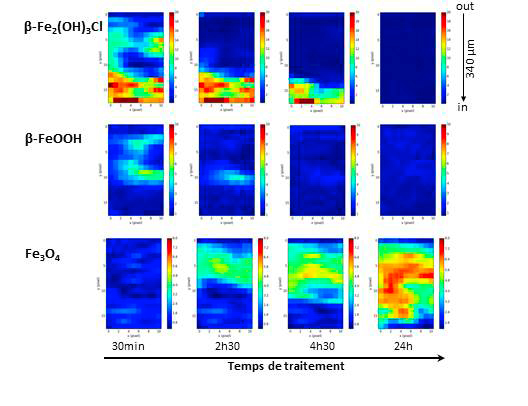
The exposed process
A specific chemical cell was designed in order to identify in situ the phases obtained by the transformation of the corrosion product layers during the dechlorination processes. The cell contains a sample from an iron bar, consisting of metal and corrosion product layers, and either aerated or deaerated chemical solution of NaOH (soda) circulating on the surface of the corrosion product layer. The in situ monitoring of the structural evolution of the corrosion system is carried out on the DIFFABS beamline: micro-beam X-ray diffraction maps reveal the distribution of the various corrosion products on a micrometric scale and their transformation during the treatment.
The initial corrosion system resulting from the iron corrosion processes in the marine environment is mainly composed of iron hydroxychloroxide β-Fe2(OH)3Cl. It has been established that this phase is rapidly dissolved by the alkaline chemical treatment solution. Regardless of the oxygen content of the treatment solution, relatively stable corrosion products in solution are formed, such as magnetite (Fe3O4) and ferrous hydroxide Fe(OH)2. However, in an aerated solution, the precipitation of a transition compound called "green rust", mixed-valence states of ferrous hydroxychloride FeII3FeIII(OH)8Cl · 2H2O is locally observed. This extremely unstable product is present only during the process in solution, as it is transformed into other corrosion products at the end of the experiment. This compound may be converted into akaganeite, implying a potential danger as to the actual stabilisation of the treated object, since this implies that a residue of the chlorinated phase remains in the corrosion products layer. On the contrary, the use of a de aerated solution leads to the formation of much more stable corrosion products and prevents the formation of intermediate and residual chlorine-containing compounds which may potentially participate in unexpected corrosion recoveries.
This study highlighted the physical processes of the stabilisation treatments of iron artefacts in order to optimise them by controlling parameters such as the aeration of the treatment solution. The conclusion of this study leads us to recommend the stabilisation of artefacts by using a de aerated treatment medium whenever possible within the conservation workshops, in order to ensure long-term conservation of the metal and consequently the collection.