Porous nanoparticles film activation for CO2 and methanol adsorption
Porous metal-organic frameworks (MOFs) are hybrid materials that can be specifically designed, among other features, for the selective uptake of specific molecules. This allows their use in several practical applications: separation processes, gas storage, sensing, etc. However, usually, as-synthesized MOF nanoparticles contain guest molecules, such as solvents and other chemicals, and it is necessary to remove them through an activation process before MOF use.
An international team of researchers from the Institute of Nanoscience and Materials of Aragón in Spain (INMA, CSIC and University of Zaragoza), SIRIUS beamline (SOLEIL), the Paris NanoSciences Institute (INSP, Sorbonne University) and the Institute of Porous Materials of Paris (ENS Paris) have used Total Reflection X-Ray Fluorescence (TXRF) to study the kinetics of a solvent-exchange process (a well-known step used in different methods for effective MOF activation) on MOF nanoparticle films.
In situ synchrotron characterization allowed to investigate the water-chloroform solvent exchange process that takes place at the air-water interface and allows optimizing film activation, increasing the CO2 adsorption capacity of MOF films by 30%. The results are published in the Journal of Colloid and Interface Science.
In this work, two crucial aspects for the development of practical applications based on nanoparticles (NPs) of metal-organic frameworks (MOFs) have been studied: the formation of dense and ordered MOF films using NPs of different sizes and the activation of these films for their use in CO2 and methanol adsorption.
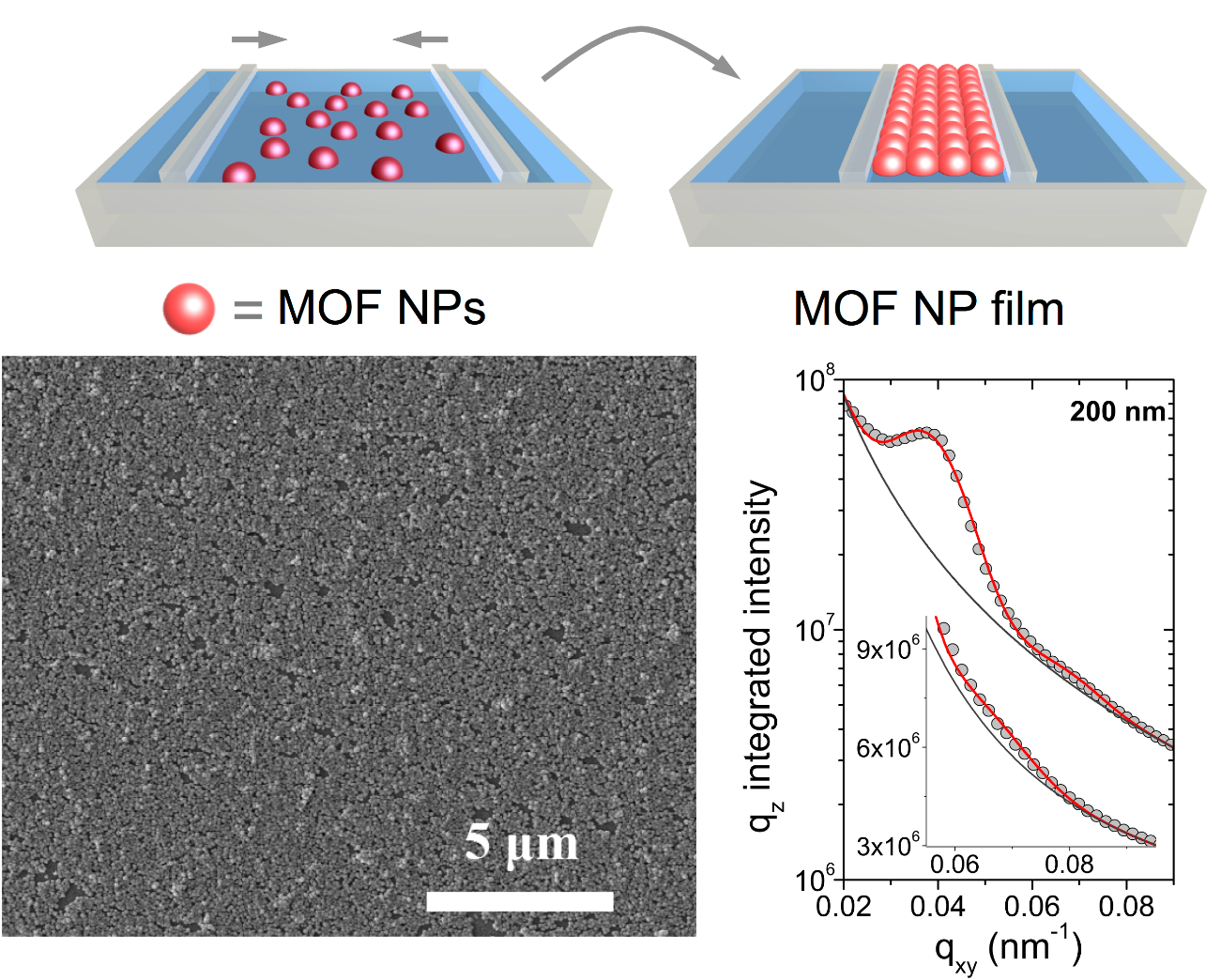
The Langmuir-Blodgett (LB) technique is a very useful method for the fabrication of compact and dense monolayer films of MOF NPs at the air-water interface that can be deposited as uniform coatings onto substrates of different nature and shape, such as quartz, mica, solid state interdigitated electrodes and fabrics containing textile electrodes (Figure 1). In particular, previous studies have shown that LB films of the microporous aluminum trimesate MOF named MIL-96(Al) (MIL stands for Materials from Institut Lavoisier) can be used as active layer of methanol and humidity sensors and also for the study of CO2 adsorption and desorption processes.
Top: schematic of MOF NP film formation at the air-water interface by the LB method.
Bottom: SEM image of a LB monolayer deposited onto a glass substrate and in situ characterization of the MOF NP film at the air-water interface at SIRIUS beamline by GISAXS, revealing a structured film.
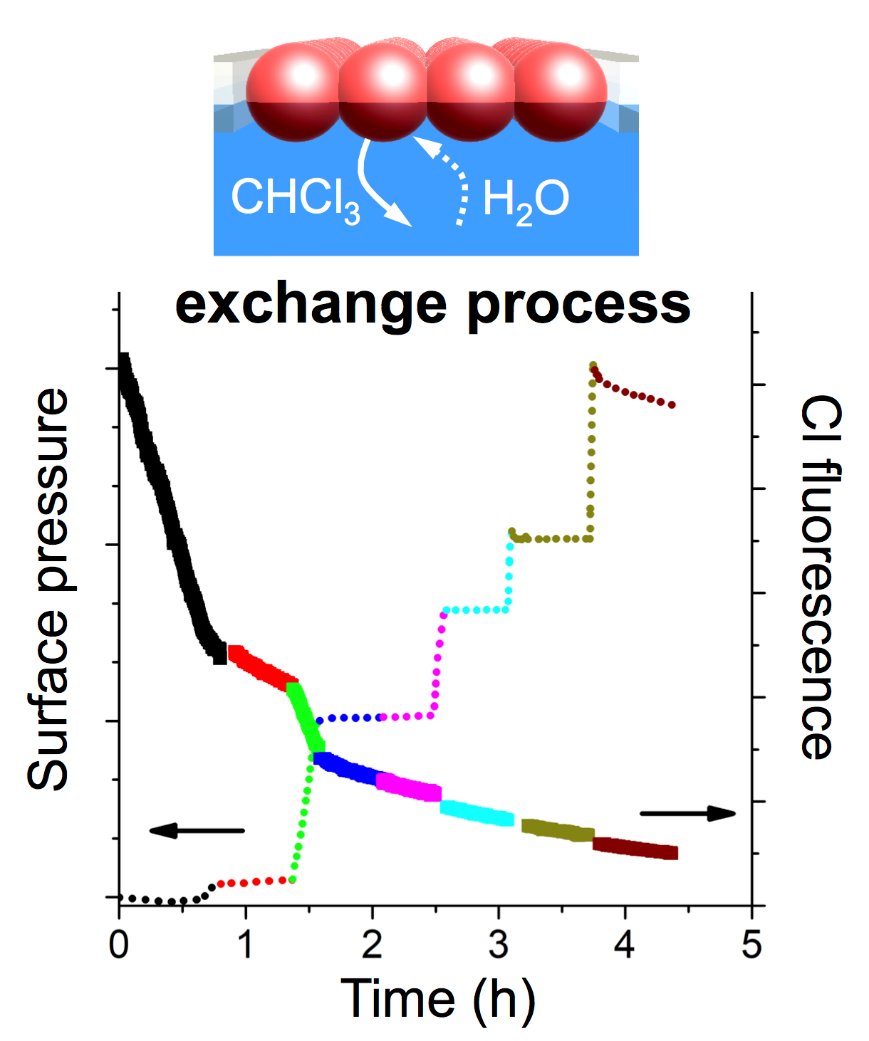
SIRIUS beamline at SOLEIL is one of the few facilities in the world that allows in situ characterization of monolayer films at the air-water interface. In this work, Grazing-Incidence X-Ray Diffraction (GIXD) have proved that MOF NPs maintain their internal crystallinity during the formation of dense and homogeneous 2D films and Grazing-Incidence Small-Angle X-Ray Scattering (GISAXS) showed that the NPs are organized. Moreover, Total Reflection X-ray Fluorescence (TXRF) has been used to study the kinetics of the chloroform exchange at the air-water interface. The experiments performed confirmed that the observed chlorine Kα signal comes from chloroform retained within the particles that continuously decreases during film formation (Figure 2). A biexponential model, characterized by a short (τ1) and a significantly longer (τ2) characteristic times, which are ascribed to, respectively, the solvent-exchange process of weakly bound chloroform molecules and the progressive removal of strongly bound chloroform molecules has been used to analyze experimental data.
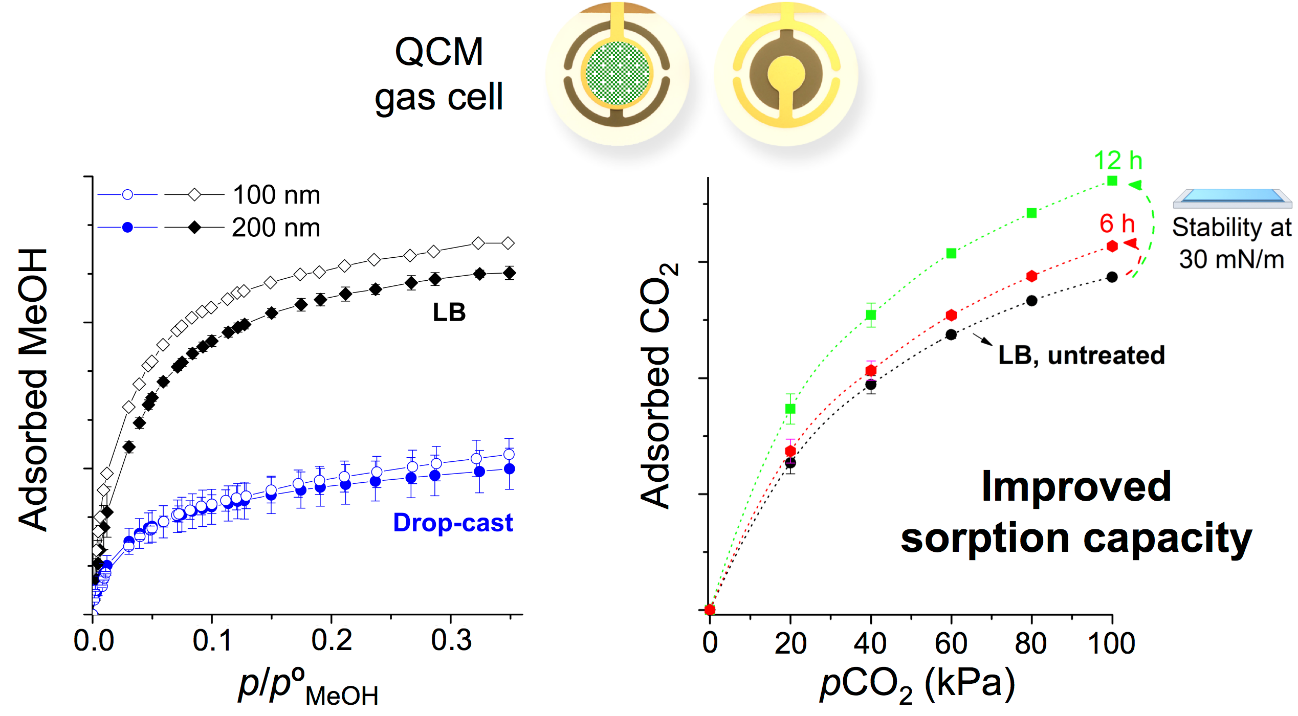
The conclusions derived from this model allowed optimizing the activation of MOF LB films, that require different exchange times, depending on the NP size. The activation can be performed either in situ (monolayers are left at the air-water interface before film deposition) or ex situ (LB films deposited onto Quartz Crystal Microbalance (QCM) sensors are subsequently immersed into water) leading to similar CO2 adsorption values. Moreover, the film ordering achieved with the LB method also increases methanol adsorption on the films, compared to other simple methodologies, such as drop-casting (Figure 3). This study, thus, demonstrates the interest of the LB technique for the study of gas and volatile organic compounds adsorption-desorption processes and for the development of gas sensors based on MOF films.
Top: schematic of Quartz Crystal Microbalance (QCM) sensors used for CO2 / methanol adsorption studies (uncovered and covered with a MOF monolayer).
Bottom: comparison of methanol adsorption on LB and drop-cast films and study of CO2 adsorption on untreated and activated LB films.