Plant oils and their derivatives. Structural study of an oleosin, a protein at the heart of energetic and medical issues
In the current context, with fossil fuels running out and high priority of environmental protection, the valorization of oil from biomass for energy and green chemistry is of increasing interest. The goal is to replace products of fossil origin with these oils and their biodegradable derivatives.
The DYSCOL team (Dynamique et Structure des Corps Lipidiques) at the Institut Jean-Pierre Bourgin (INRA-AgroParisTech, Versailles) works to identify factors affecting the quality and quantity of oils produced in plants and microorganisms and to promote the development of more efficient and more environment-friendly oil extraction processes. At DISCO beamline, the circular dichroism technique allowed them to obtain, in conditions close to the physiological ones, data about the secondary structure of an oleosin. This protein is involved in the storage of oils in oleaginous plants
Considering the valorisation of biomass oils for energy and green chemistry, two sources are under consideration: plant oils, which are already well established, and oils produced by microorganisms, whose use is rapidly expending. These oils are more and more present in ordinary consumer goods (biodiesel, soap, cleaning products) and industrial products (solvents, lubricants).
Oils, oil bodies, and green chemistry
In cells, oils are stored in granules: the oil bodies. These structures are present in higher eukaryotes (mammals and plants) and in some microorganisms (bacteria, yeasts). Oil bodies comprise an oil core surrounded by a mono layer of phospholipids where many proteins are inserted. In particular, there are hydrophobic structural proteins that stabilize the interface between these lipid inclusions and the aqueous medium (perilipins, apolipoproteins, oleosins). Oleosins, with their mass between 15 and 25 kDa, are the major proteins of plant oil bodies, and completely cover the oil body surface.
The structure of oleosins: an economic and medical issue
Besides their importance for stabilising the fat deposits in plant, peanut oleosins are allergenic proteins. More generally, oil bodies, which are also present in mammals, play a major role in diseases that are more and more widespread (atherosclerosis, obesity, diabetes). For these reasons, understanding structures of oleosin and all the oil-body integral proteins, is of major importance for the agricultural, economic, and medical field.
A first: circular dichroism on lipid nanoparticles in aqueous solution
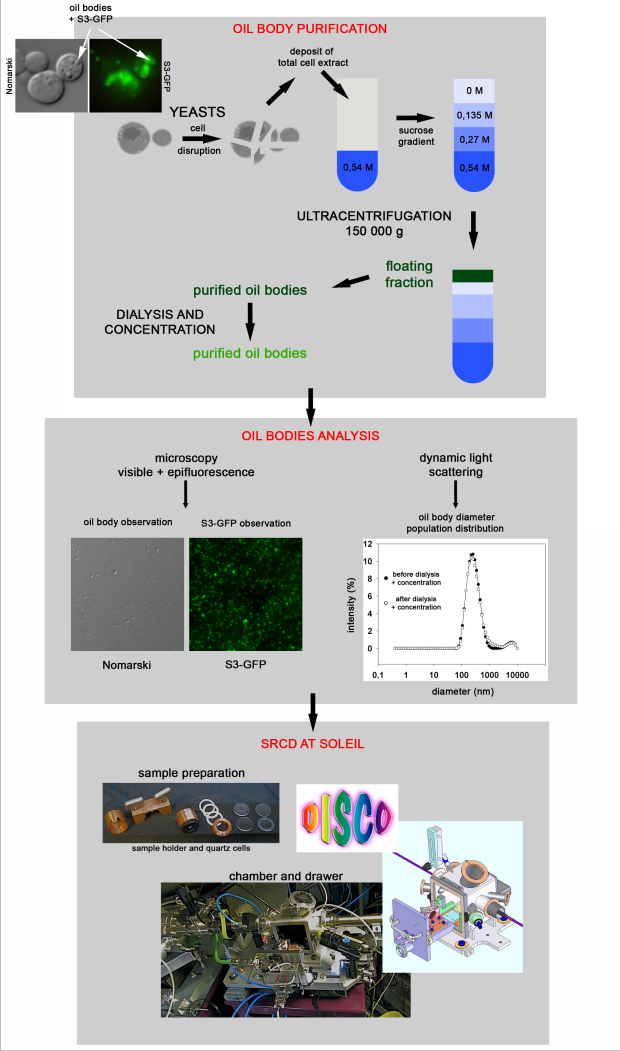
The DYSCOL team uses the “baker’s yeast” (S. cerevisiae) as a model cellsystem to perform a structural study of oil bodies and their integral proteins: the scientists engineered this yeast –that “grows” easily in laboratory- to produce vegetable proteins (heterologous expression). This production is followed by cellular fractionation, in order to separate the yeast components (see fig. 1). This allowed obtaining oil bodies compatible with structural analysis.
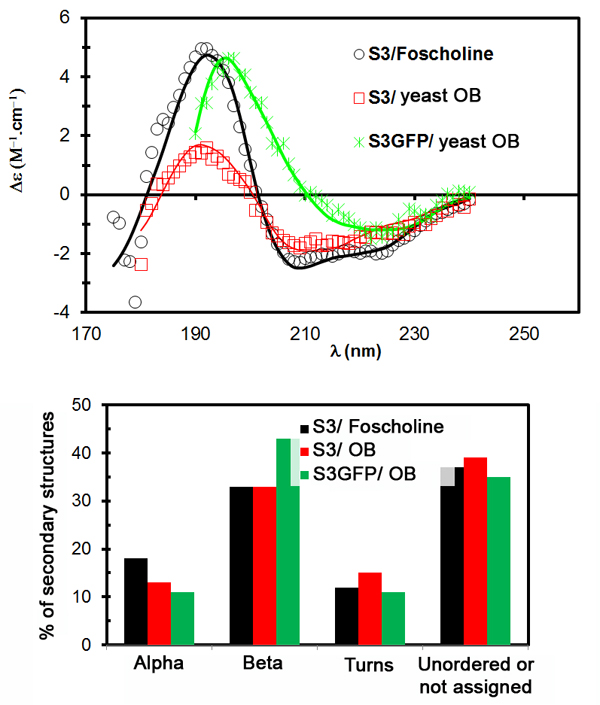
Thanks to this original approach, SRCD (circular dichroism using synchrotron radiation) spectra on oil bodies in solution were recorded (cf fig.2). For the first time, SRCD spectra were collected on proteins incorporated in oil body nanoparticles (250 nm), held in aqueous solution. This medium is closer to physiological conditions (i.e. natural environment of proteins in the cell) than surfactants usually used to solubilize proteins for structural studies.
The acquisition of spectra in a turbid medium is possible thanks to the brightness of the synchrotron beam and the use of UV-compatible buffers. The acquisitions were performed in Suprasil (Hellma) quartz cells with an optical path of 100, 200 and 500 µm. Spectrum processing and determination of secondary structures were performed using CDtool software and the ContinLL algorithm available online on DICHROWEB.
Thus, the folding of oleosin S3 from the plant Arabidopsis thaliana was determined in its native environment, the oil body. The results reveal a high content of beta sheets (cf fig.2). The same folding is obtained in a detergent similar to a phospholipid: Foscholine 12, validating the use of this detergent for further high resolution structural analysis (cristallography).
With these data, it was possible to suggest a folding model for this protein. These results have been published in the journal BBA – Biomembranes.
The adventure continues, still at SOLEIL
The DYSCOL team is now workingon the DISCO beamline to obtain structural data on other oleosins, as well as oil-body integral proteins from all living organisms.
In addition, other structural analysis methods are performed: infrared absorption spectroscopy (sFTIR), small-angle x-ray scattering (SAXS), structural proteomics, at the SOLEIL beamlines SMIS, SWING and METROLOGIE.
Reference :
"High water solubility and fold in amphipols of proteins with large hydrophobic regions: oleosins and caleosin from seed lipid bodies"
Gohon, Y., Vindigni, J. D., Pallier, A., Wien, F., Celia, H., Giuliani, A., Tribet, C., Chardot, T., Briozzo, P. (2011) BBA-Biomembranes, 1808(3): 706-716