The patinas of Lunéville château sandstones under the SOLEIL X-rays
Lunéville château, aka the "Versailles of Lorraine", built in sandstone of the region, has had an eventful history marked by several fires. The most recent one, in 2003, was devastating, destroying almost the entire roof of one wing of the building. During the restoration work that followed, black spots were discovered on some of the stones. These non-cleanable stains, called patinas, are rich in manganese (Mn), and their formation seems to be more related to the water brought by the firemen than to the flames of the fire. To be sure, fine laboratory characterizations are necessary.
The FLB company, in charge of part of the restoration of the castle, and the laboratories LRMH, LGE, ISTerre and ISTO have initiated a research project to better understand the mechanisms of formation of these patinas. They are present in wet areas, exposed or not to the 2003 fire. The characterization of the materials (sandstones and patinas), major axis of this work, required the assistance of the DiffAbs and LUCIA beamlines at SOLEIL for a better description of the Mn-bearing phases.
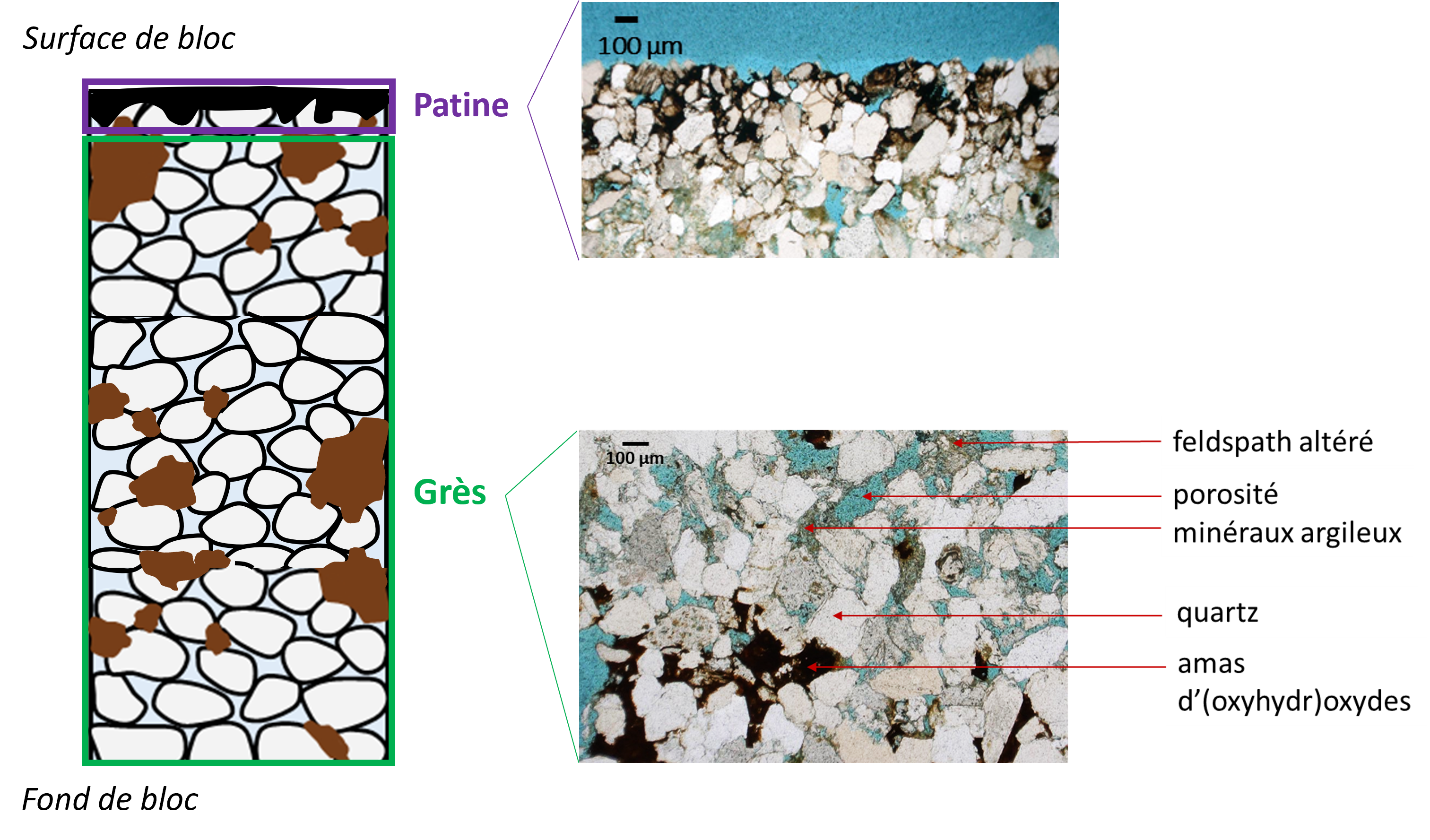
The sandstones of the Lunéville château are porous and permeable materials, light in color, made up of different minerals (Figure 1).
On the left, schematic representation of the patinated sandstones of the Lunéville château.
On the right, two images obtained by polarizing optical microscope (polarized natural light): a patina (top) and a sandstone (bottom).
Between the grains of quartz and feldspar are disseminated smaller particles: clays and clusters of (oxyhydr)oxides. The latter correspond to "dark spots" visible to the naked eye, and analyses by optical and electron microscopies have highlighted their enrichment in Mn, with sometimes iron (Fe) or barium (Ba). The patinas (figure 1), on the other hand, are only enriched in Mn and do not form a uniform layer on the surface of the stones, but rather consist of manganese phases embedded between the grains of the sandstone. Patinas formed "naturally" (outside of the fire and firefighters' spray) can be distinguished from those formed as a result of the fire, by their darker color and greater depth.
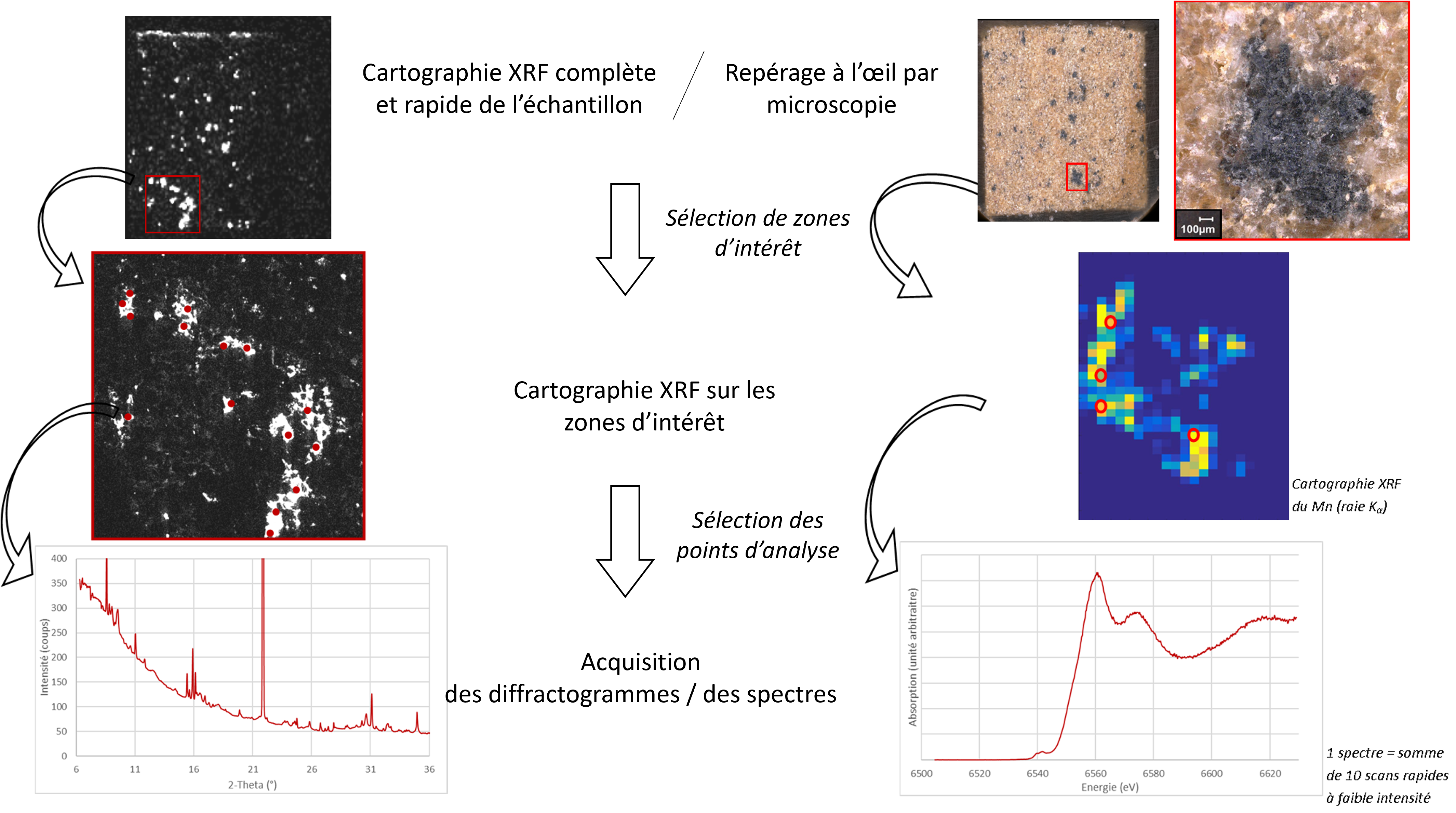
There are more than fifty different Mn oxides, so it is relevant to go further in the characterization, but the weak crystallinity and the fragility of these minerals with respect to certain radiations make their identification complex. Moreover, for the analysis of small and not very concentrated zones, it is necessary to use techniques equipped with a micro-beam. This is why it was chosen to combine Raman micro-spectrometry (in the laboratory), X-ray micro-diffraction and X-ray absorption micro-spectrometry at the Mn K-edge -on the DiffAbs and LUCIA beamlines, respectively- to study the Mn bearing phases in the "dark spots" of the sandstone and in the patinas. The same analytical approach was followed for the three techniques (Figure 2): Mn-rich zones were chosen on visual criteria, at different distances from the surface in order to highlight a possible evolution in the oxidation state or the mineralogy of Mn, and then several measurements were made. Special precautions were taken to avoid interactions with the beams before the analyses in order not to degrade the minerals.
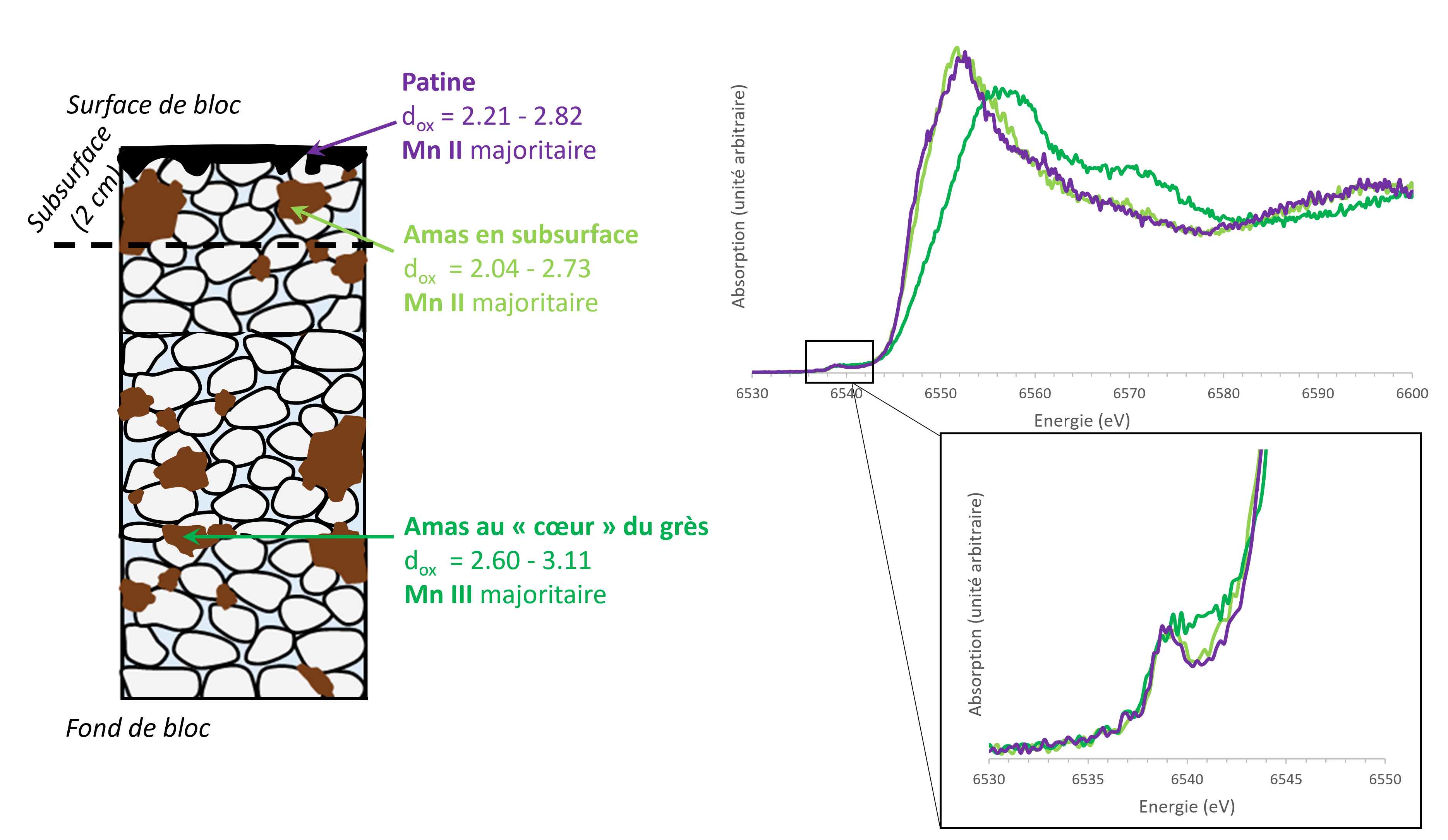
The results obtained complement each other and have provided key information. In the case of a "naturally formed" patina, distinct Mn oxides could be identified in the patina (birnessite) and in the underlying sandstone (hollandite). For the patina formed after the fire, the identification of Mn oxides was not possible, but the study of the oxidation states brought another important result (Figure 3): the closer the Mn is to the surface, the lower its degree of oxidation. This could be an evidence of a dissolution by fire water of the Mn oxides initially present in the sandstone.
Several other clues suggest that the colored clusters in the sandstone are the source of Mn for the formation of the castle patinas. In particular, dynamic laboratory tests have shown that this internal Mn was mobilizable by water and could produce a browning on the stone. All the results obtained allow us to propose a model of patina formation based on the dissolution of the manganese phases initially present in the "dark spots" inside the sandstone, then the migration of the ions released in solution towards the surface of the stones where they precipitate.
On the left, schematic representation of the X-ray absorption micro-spectrometry results for the area of the castle affected by the 2003 fire.
On the right, examples of spectra obtained in the core of the block, in the subsurface and in the patina.