Near-infrared for improved detection of luminescent nano-compounds in living cells
Fluorescence imaging is an emerging technique for biomedical applications. It can be used to observe and track a specific target (constituents of the cell, pathogen, active ingredient, etc.) in real time and in a non-invasive manner. Using the DISCO beamline equipments, French and American teams have developed a new non-toxic fluorescent marker which allows increasing detection sensitivity considerably.
The main limitation is the intrinsic fluorescence of the biological components (autofluorescence), which interferes with the signal emitted by the imaging agents. This effect can be avoided by using near-infrared light. This type of light has less interaction with the components of tissues, so that image quality can be improved and detection sensitivity can be increased. There are currently very few efficient fluorescent markers for biological imaging in the near-infrared. The few agents that are commercially available are highly sensitive to light (photobleaching) or are relatively toxic.
Most lanthanide-based molecules emit a very weak fluorescence signal in the near-infrared range, which means that they cannot be used for imaging. The challenge in this case was to develop a compound whose structure would allow the number of lanthanides per unit volume to be multiplied, thus considerably increasing detection sensitivity.
Porous materials called MOFs (Metal-Organic Frameworks) have allowed significant fluorescence of lanthanides to be obtained in the near-infrared range. It has been shown that these luminescent lanthanide-based compounds have low toxicity and good resistance in water, an essential element for biological applications.
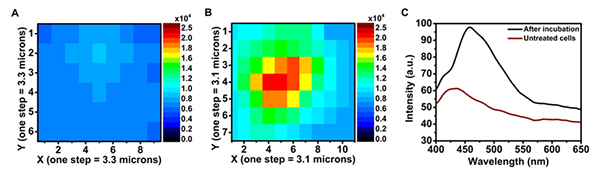
The synthesized nano-MOF- Yb-PVDC-3 absorbs in the UV range and emits in the visible (450 nm) and near-infrared (980 nm) ranges. Because it was not technically possible to perform confocal microscopy in the near-infrared, the emitted signal was collected in the visible range only. The images are recorded in a spectral zone where the autofluorescence is very present, and are not sufficient to confirm the presence of our compound in the cells. The spectral microscopy performed on the Polyphème microscope of the DISCO beamline allowed to unambiguously distinguish the autofluorescence signal of the cells from the signal emitted by the nano-MOF- Yb-PVDC-3. (Figure 1.)
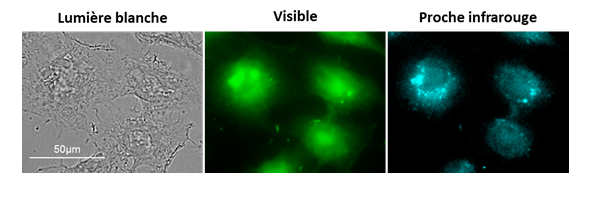
The strategy developed allowed us to obtain the first microscopy images using luminescent lanthanide-based compounds emitting in the near-infrared range in living cells (Figure 2).
This work was the product of multi-disciplinary research at the interface between chemistry, biology, and physics. The initial results are very promising for the development of imaging agents that will be effective in the near-infrared range, for use in biological research and ultimately for clinical applications.