Multifunctional materials. Structural approach of the pseudocapacitive properties of Cobalt containing layered double hydroxides
With numerous possible applications in fields such as energy, the environment, health or polymers, layered double hydroxides (LDH), which are particularly suitable for the development of multifunctional materials, are currently the subject of significant research.
With the aim of controlling the performance of these materials, several studies performed recently by the Inorganic Materials team of the Institute of Chemistry of Clermont-Ferrand (ICCF) are based on use of synchrotron radiation. Their last results obtained notably on the SOLEIL CRISTAL beamline have been published in the journal Chemistry of Materials.
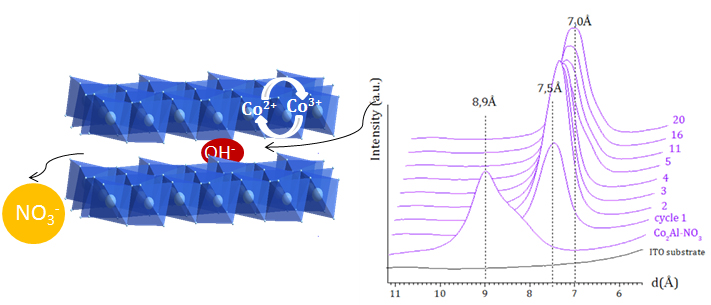
Layered double hydroxide (LDH) materials have the general formula [M2+1−x M3+x(OH)2][Xm−x/m,nH2O]. Not only their variety of composition both in respect of the metals (M2+, M3+) that constitute the hydroxide layers as well as in respect of the intercalated species Xm−), but also their high anionic exchange capacity renders them candidates particularly suited to the preparation of multifunctional materials. The numerous synthesis paths and possibilities for shaping these materials must also be added to this chemical and structural flexibility, which increases their range of application.
Control of the performances of these materials requires an ever more detailed understanding of their structure as well as the relationships between the synthesis/structure (composition)/usage properties.
To do this, various aspects of the LDH phase structure have been studied by the Inorganic materials (ICCF) team using X-ray diffraction and scattering measurements, recorded at large Q (scattering vector) on the CRISTAL beamline: the average structure using the Rietveld method, the microstructure by analysis of the profile of Bragg diffraction peaks, the structural disorder and the presence of defects by the pair distribution function (PDF)analysis of diffuse scattering This work has expanded what is known about the LDH phase structure, more precisely addressed the structural description and improved the understanding of the microstructure of these poorly crystallised materials.
LDH materials developed for the conversion and storage of energy
The presence between the LDH layers of electroactive cations such as Ni2+, Co2+ and/or Co3+ is the source of remarkable pseudocapacitive properties. Thus cobalt-based compounds can display a charge capacity of up to 1500 F g-1.
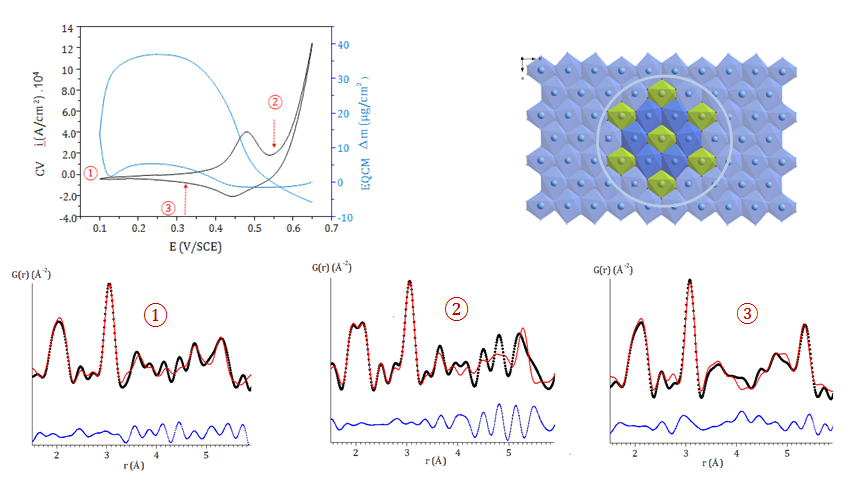
A combined approach of ex-situ and operando measurements was applied, which made it possible to report on both structural changes and redox processes occurring within these materials in accordance with the electrochemical behaviour characteristics. The measurements were made on the CRISTAL 2-circle diffractometer with the multi-crystal analyser for the ex-situ measurements and the hybrid pixel detector for the operando measurements; a monochromatic beam of wavelength λ=0.4368Å (28,2518 keV) was used providing access to a wide range of Q: Qmax =25.4 Å–1. In addition, an electrochemical cell designed for carrying out operando measurements using X-ray grazing incidence diffraction was employed.
Operando measurements over a Co2Al phase during a cyclic voltammetry (CV) experiment were carried out on a thin film deposited on an ITO electrode in the KOH electrolyte. These have made it possible to reveal an ion exchange dynamic in the inter-layer space of the LDH phase, concomitant with the transfer of electrons within the hydroxide layers. These movements of chemical species at the LDH/electrolyte interface could be quantified using complementary electrochemical quartz microbalance (EQCM) measurements.
In addition, high quality total X-ray scattering data were recorded for oxidised and reduced samples prepared ex-situ by electrolysis. The structural analysis using the pair distribution function indicates the existence of significant disorder in the hydroxide layers which appears from the second coordination sphere around the cations r> 5-6 Å, including for the pristine material. The structural changes accompanying the changes in oxidation state of the cobalt within these layers could be characterised by refinement of the PDF over a short range. These refinements show that the coexistence on the same crystallographic site of Al and Co species is favourable for the electrochemical reversibility of the cobalt oxidation.
The nanostructure and the disorder which characterise the LDH phases are therefore an integral part of the mechanism for accommodating the changes in oxidation state of electroactive cations in these materials, accounting for their pseudocapacitive performance.
Acknowledgements
The authors would like to thank P. Vialat, S. Peulon (UMR 8587 CNRS - Lambe, University of Évry) and H. Perrot (UMR 8235 CNRS - Lise, UPMC) for their essential contribution to this work as well as E. Elkaim (SOLEIL, Gif-sur-Yvette) for the valuable assistance during the synchrotron experiments.