Molecular mechanism of LDL cholesterol regulation
In a crucial step towards understanding the mechanisms of cardiovascular disease and certain cancers, a research team has achieved a world first: finding the molecular mechanism by which the PCSK9 protein degrades the receptors for LDL - low-density lipoproteins - the molecules in the blood that transport cholesterol to the cells that use it. The degradation of LDL receptors leads to an increase in the blood level of LDL-cholesterol, which are the most abundant cholesterol particles in the blood. This degradation by PCSK9 can be inhibited by a mini antibody (nanobody) named P1.40.
The use of such therapeutic inhibitors could reduce the risk of atherosclerosis and heart disease.
Despite its bad reputation, cholesterol is a molecule -of the lipid family- indispensable to our body: role in the propagation of nerve impulses, component of cell membranes, precursor of hormones and vitamins... cholesterol is essential. Its transport in the blood is ensured by several types of molecules of the lipoprotein family. High-density lipoproteins (HDL) transport the cholesterol that has been "used", i.e. oxidized, and bring it back from the arteries or tissues to the liver, where it is degraded; this is what we call "good" cholesterol.
Low-density lipoproteins (LDL) transport cholesterol from the tissues where it is secreted - mainly the liver and intestine - to the cells of the body that need it. An accumulation of LDL in the blood can lead to atherosclerosis and heart disease - hence the name "bad cholesterol" for LDL.
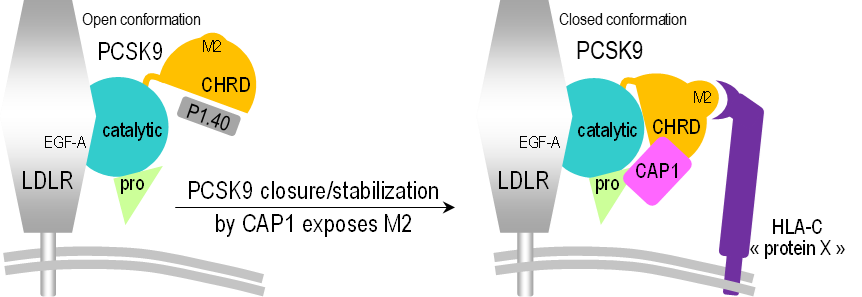
On the surface of cells that need cholesterol are receptors to which LDL binds before entering the cells where the cholesterol is used. Once LDL is in the cell, the receptors return to the cell surface to continue their function.
If, due to a malfunction, LDL is not captured by the cells, it will accumulate in the blood with the risks mentioned above. Such a dysfunction may be due to a problem with the LDL receptors or, in some cases, with a protein called PCSK9.
Secreted into the bloodstream by the liver, PCSK9 helps liver cells degradate LDL receptors. Overactivity of PCSK9 results in a decrease in the number of functional receptors on the surface of the cells, and therefore in the amount of LDL captured, increasing the level of LDL in the blood. Some hypercholesterolemia are thus due to the presence of overactive PCSK9; this protein is therefore a subject of choice for research on this type of pathology.
Carole Fruchart Gaillard and her colleagues from the Department of Drugs and Technologies for Health (CEA/UPSaclay, Gif-sur-Yvette), together with scientists from the Neuroendocrine Biochemistry Research Unit (IRCM, Montreal), the Department of Pharmacy at the University of Pisa in Italy and the SWING and PROXIMA-1 beamlines at SOLEIL, have studied the previously unknown mechanism involving PCSK9 that leads to the degradation of LDL receptors.
In this work, they used a small synthetic protein called P1.40 which, in previous studies, has been shown to inhibit the degradation of PCSK9. Using small angle X-ray scattering on the SWING beamline, a structural change of the PCSK9 protein in solution was observed, which depends on the presence or absence of P1.40. The latter is inserted between two areas of PCSK9 (called CHRD domain and catalytic domain), thus preventing PCSK9 from taking its active form.
On the PROXIMA-1 beamline, the details of this inhibition mechanism were clarified by an X-ray diffraction study carried out on crystals of the CHRD/P1.40 complex. This study allowed to determine the 3D structure of CHRD/P1.40 at atomic resolution.
Their work also focuses on other molecules involved in the degradation process of LDL receptors. One of them is a key protein of the immune system, HLA-C, which has been shown to play an essential role: linked to other proteins, including PCSK9, it directs the receptor towards the lysosomes which will degrade all these different proteins.
Inhibiting PCSK9 would then prevent the degradation of the various related proteins (see above), which would then increase the amount of HLA-C on the surface of the cells. HLA-C allows the recognition of "self" and stimulates the antitumor activity of T cells, hence the protective effect of PCSK9 inhibition against tumor growth and associated metastasis.
Ultimately, the hope is to develop inhibitors that would prevent the interaction of PCSK9 and HLA-C and block the function of PCSK9 on LDLR and HLA-C.
This breakthrough could then be applied to treat cardiovascular disease as well as various types of cancers and metastases.