Of mice and men… and uric acid
In humans an excess of uric acid (or hyperuricemia) is associated with, or responsible for several serious health problems: metabolic syndrome1, gout and kidney dysfunction. Other living beings are not subject to hyperuricemia because they possess an enzyme, absent in hominids, that degrades uric acid. To increase our understanding of the role of uric acid in these diseases, researchers at the University of Lausanne, UPMC and Tenon Hospital blocked the degradation of uric acid in a mouse strain, and studied the impact of acutely further increasing hyperuricemia in mice fed a normal, or an obesogenic, high fat diet. In the hyperuricemic mouse strain, high fat feeding combined with the hyperuricemiant dietary treatment to induce acute renal failure, due to the accumulation of urate and uric acid crystals in the kidneys. Infrared microscopy on the SMIS beamline was used to analyze the composition of crystals. Their results should motivate us to pay more attention to our diet.
Uric acid is a degradation product of purines, which are components of DNA, and are provided in the diet primarily from meat and fish. In healthy humans, uric acid is excreted in the urine and present in relatively high amounts in the blood (plasma).
Uric acid: advantages and disadvantages
With the help of the enzyme urate oxidase or uricase, all organisms, from bacteria to mammals, are capable of degrading uric acid, which is therefore not the "final waste product" in purine catabolism. All organisms... except higher primates, and therefore humans.
During evolution hominids lost the ability to produce uricase. It has been suggested (without direct evidence so far) that uric acid, with its powerful antioxidant property, has a protective effect on the body, resulting in a longer lifespan and a decrease in the levels of age-related cancers.
However, uric acid does not only have positive effects, making the hypothesis of advantages linked to the loss of production of uricase during evolution rather controversial. Indeed, when present in excess (hyperuricemia), uric acid exposes the body to serious health problems: gout, renal dysfunction and (highly controversially) certain aspects of metabolic syndrome1. However hyperuricemia alone does not cause the different physiological problems cited.
Understanding the effects of an excess of uric acid
In order to study the influence of hyperuricemia on health, researchers developed an animal model that, like humans, does not degrade uric acid: a mouse line in which the elimination of uric acid was prevented by inactivation of the GLUT9 gene in the liver (LGLUT9-knockout mice), which encodes a key transporter protein of uric acid metabolism.
Initially, four cohorts were studied: mice with and without this transporter and fed with either a normal, or a high fat diet.
In all LGLUT9-knockout mice, blood uric acid levels were high, but never reached the values measured in humans. Apart from the obesity caused by a coconut-oil diet, they remained in good health: no kidney problems were found.
Fat-rich diet and excess uric acid, a losing combination
To get closer to human uric acid levels, the diet of some mice was supplemented with inosine, the precursor molecule of uric acid.
Without effect in control mice (with GLUT9), the addition of inosine actually increased uric acid levels in deficient mice. Among them, the animals fed a balanced diet quickly returned to normal after a few hours, although they ingested inosine for 3 days. Blood and kidney tests confirmed that in these animals, blood uric acid levels increased transiently without causing major problems.
On the other hand, the uric acid levels in obese mice eating a high fat diet increased after 3 days of inosine consumption. Renal function in deficient mice was impaired: the formation of crystals blocked renal tubules, preventing the evacuation of urine (renal failure) and the urate no longer being discharged via urine had accumulated in the blood.
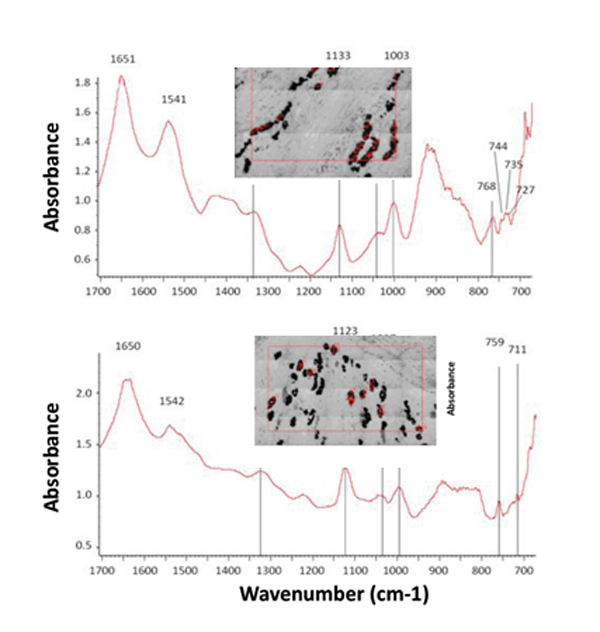
These crystals, when studied on the SMIS beamline were found to be much bigger and more plentiful in obese mice than in mice on healthy diets (control mice had no crystals at all). The infrared microspectroscopy technique, performed on kidney sections, made it possible to distinguish several types of crystals of uric acid or urate, the ion derived from uric acid.
Among the conditions that predispose to crystals formation, acidic urine is the main factor. In L GLUT9-knockout mice, high fat feeding resulted in a more acidic urine, thus predisposing to crystal fomration. This factor could, in combination with hyperuricemia, cause these crystals to form, resulting in the loss of renal function in high fat-fed mice.
A reversible effect, but with long-term consequences
Several weeks after stopping the intake of inosine, the researchers again studied the blood and kidneys of these mice. It became clear that, although the animals had recovered from the kidney failure and the crystals had disappeared, the tissue damage caused by these crystals had led to long-term consequences: kidney inflammation, fibrosis, etc. These effects were naturally found to be much more severe and widespread in obese mice.
These different findings in mice are consistent with the epidemiological data suggesting that obesity plays a role in hyperuricemia.Uric acid is a molecule whose role remains complex and partly unknown, and in particular its anti-oxidant properties which, under certain cell conditions, can switch and become pro-oxidant and promote inflammation.
By combining a genetic modification and the use of inosine, researchers were able to obtain an animal model with which to study the long-term role of hyperuricemia in the development of diseases such as hypertension or atherosclerosis.
1 – metabolic syndrome: a set of conditions (partly related to genetic factors, but mainly due to the environment: activity and nutrition), which, when combined, increase the risk of developing cardiovascular disease and diabetes.