Lithium ion batteries : Polythiophene coordination complexes, a promising material
Current lithium ion battery (LIB) technologies are all based on inorganic electrodes, though organic materials have been hyped as electrodes for years. Disadvantages such as low specific capacity and poor rate performance hinder their applications.
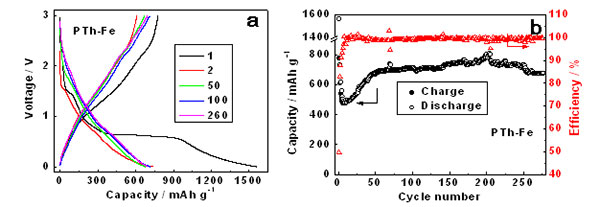
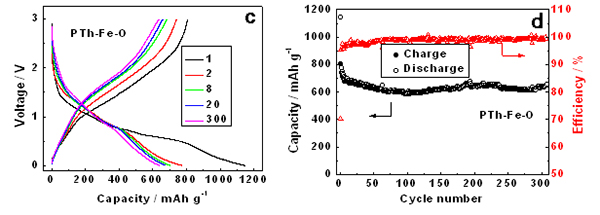
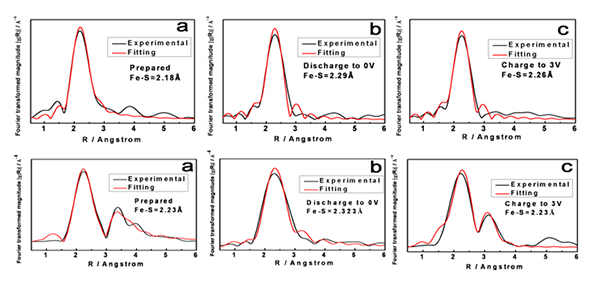
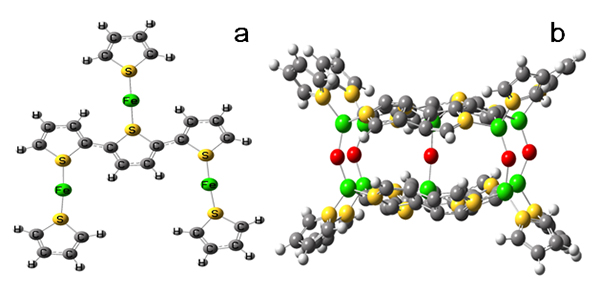
Scientists from Beijing China and SOLEIL France (Beamlines LUCIA and ODE) revealed that chemically synthesized organometallic polythiophene-iron and polythiophene-iron-oxygen complexes are promising lithium storage materials with high reversible capacity and excellent cycling stability. Combined studies of extended X-ray absorption fine structure (EXAFS) spectroscopy and density functional theory (DFT) illustrate that the iron metal atom is incorporated into the polythiophene network forming strong Fe-S bonds, which endows the polymer with high specific lithium storage capacity. In addition, the high reversibility of its interlayer Fe–O–Fe interaction during cycling ensures its high cycling stability while the conducting polythiophene matrix endows it with outstanding rate performance.