Hydrogen production – a promising electrocatalyst based on clay nanotubes
Hydrogen is one of the avenues explored to replace fossil fuels. Producing hydrogen by splitting water is a possible pathway, but it requires the use of catalysts that are often made of scarce, expensive materials whose extraction is not environmentally friendly. It is crucial to discover new, cost-effective, noble-metal-free catalysts that still preserve high performance.
A consortium led by researchers from the Laboratoire de Physique des Solides and the Institut de Chimie Physique (CNRS/UPSaclay) has demonstrated the potential of geo-inspired clay nanotubes as sustainable electrocatalysts for the oxygen evolution reaction, the bottleneck in water-splitting processes. Four SOLEIL beamlines contributed to these results.
The oxygen evolution reaction (OER), also known as the water oxidation reaction, 2H₂O ⟶ 4e⁻ + 4H⁺ + O₂, naturally occurs during photosynthesis, which produces the oxygen we breathe. This reaction involves a four-electron transfer, competes with peroxide formation, and requires catalysts to proceed. In recent years, major advances have been achieved with Ir- and Ru-based catalysts, which are considered as benchmark materials for OER. However, despite their high activity and stability, the scarcity and high cost of these elements represent significant limitations for large-scale applications compared with more Earth-abundant elements.
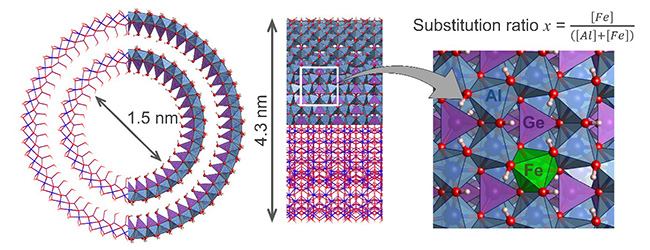
Geo-inspired nanotubes, such as germanium-based imogolite (GeAl₂O₃(OH)₄, Ge-NTI), feature a unique tubular structure and composition that make them promising materials for catalytic reactions. Moreover, selective isomorphic substitution of elements within their structure offers a simple way of tuning their conductivity and catalytic properties—while preserving the original tubular structure, which remains a major challenge.
This strategy was adopted by a national consortium involving researchers from the Universities of Paris-Saclay, Cergy and Poitiers, in collaboration with four SOLEIL beamlines. Through a one-step hydrothermal synthesis, iron atoms were successfully incorporated into the structure of double-walled germanium imogolite (Figure 1), following the general formula Ge(Al2-2xFe2x)O3(OH)4.
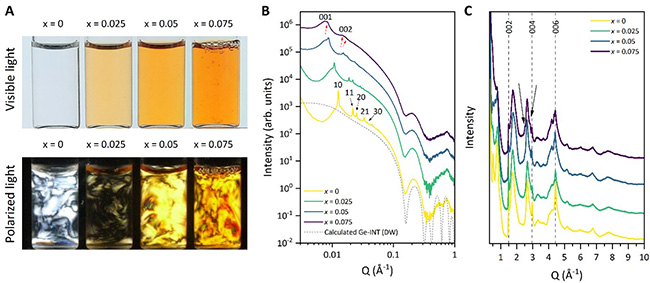
For substitution ratios x = [Fe]/([Al]+[Fe]) below 0.1, the original structural properties of the nanotubes are preserved, including their monodisperse diameter, colloidal stability, and unique self-organization behaviour. These features were confirmed by small-angle X-ray scattering on the SWING beamline and powder diffraction on the CRISTAL beamline (Figure 2).
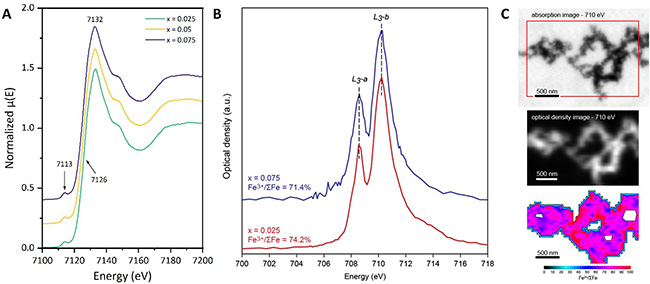
To complete this study, XANES spectroscopy performed on the LUCIA beamline confirmed the incorporation of iron atoms at octahedral sites in the Fe-doped Ge-NTIs (Figure 3A). To obtain detailed information on the crystal chemistry of the synthetic samples, the team quantified the ferric/ferrous ratio at the L₃ edge using scanning transmission X-ray microscopy (STXM-XANES) on the HERMES beamline. Nanoscale chemical mapping revealed a homogeneous distribution of Fe sites along the nanotubes, with Fe³⁺/ΣFe values consistently above 70%, regardless of the substitution ratio (Figure 3B).
The OER electrocatalytic activity of Fe-doped Ge-NTIs was evaluated under alkaline conditions using a three-electrode system. The best material, obtained for x = 0.05 iron content, shows performance comparable to that of noble-metal-based catalysts or Ni/Co oxides. The tests also demonstrate good electrochemical stability, an essential parameter for potential industrial applications.
Overall, these results highlight the promising potential of geo-inspired imogolite nanotubes as a sustainable and scalable alternative to conventional noble-metal-based OER catalysts, while opening new perspectives for the application of imogolite-based nanomaterials in electrocatalysis and renewable-energy technologies.