An intramolecular disulfide bridge regulates the affinity of human neuroglobin for oxygen.
A French group has determined the structure of human neuroglobin, highlighting a new mechanism for regulating the affinity of neuroglobin for oxygen.
Neuroglobin is a small protein in the globin family, discovered in the year 2000, which is present in the brain of vertebrates. Although its functioning in an organism is still poorly understood, it is overexpressed under hypoxia conditions (lack of oxygen), thereby protecting cells. Unlike other globins, where the sixth coordination site of the heme iron is available and can bind itself to oxygen, histidine, in a distal 64 position, is the Fe ligand of neuroglobin, both in the oxidized and reduced states. It has been suggested that this hexa-coordination is a new regulating mechanism, since here the limiting step for exogenous ligand binding is breaking the connection between the heme iron and the distal histidine.
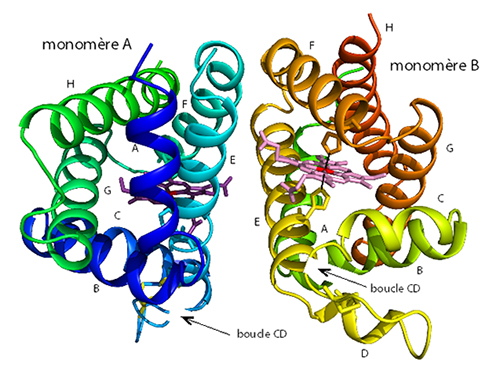
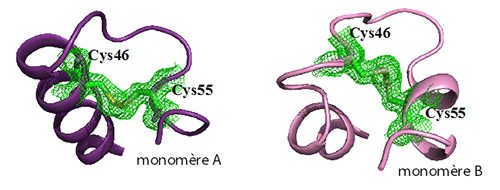
The hypothesis had been that in human neuroglobin, the formation of a disulfide bridge between the cysteines at positions 46 and 55 (present in the CD loop linking the C and D helices) would reduce the affinity of the distal histidine for heme iron, thereby increasing its oxygen affinity. However, until now, there have been no structural data showing the presence of such a disulfide bridge and thus explaining its regulatory function.
Using X-ray diffraction experiments on the PROXIMA1 beamline at SOLEIL Synchrotron, the research groups of Michael Marden (INSERM) and Beatrice Golinelli-Pimpaneau (Collège de France, CNRS, Paris) determined the structure of human neuroglobin. The crystal contains two structures of the protein in which the disulfide bridge is formed, but adopts different conformations, thus revealing a considerable flexibility. Analysis of the structure showed the formation of a new internal cavity in the CD loop region where the disulfide bridge was formed. This cavity may enable the conformational mobility of the distal histidine in human neuroglobin, and thus regulate the molecular dynamics between the hexa- and penta-coordinated forms, or even shift the route of ligand migration. This new mechanism for regulating the affinity of neuroglobin for oxygen, involving the formation of an intramolecular disulphide bridge, would be specific to mammals, with the exception of rodents.