How to obtain a clear electron image when the nucleus of an atom perturbs the ionization process
Ionization is a phenomenon scientists have been trying to study closer and closer. Unfortunately, till now they were faced with an important blurring of the image taken. An international team recently managed to obtain a clear electron image of the ionization of Xenon.
How to obtain a clear electron image when the nucleus of an atom perturbs the ionization process.
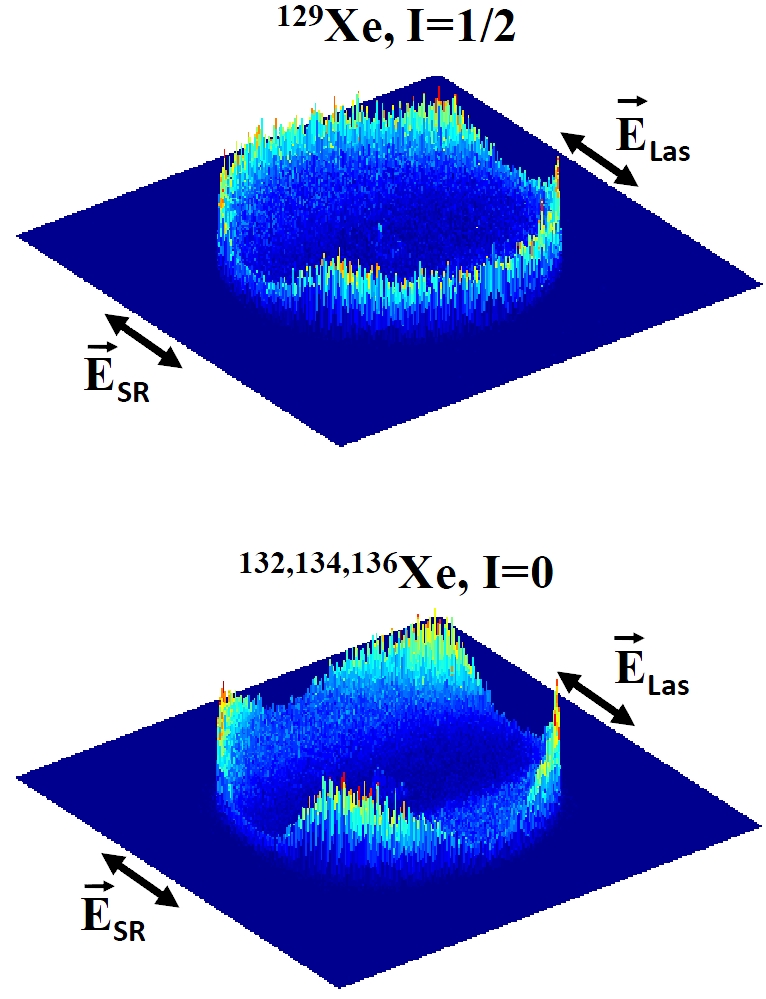
When an atom or molecule absorbs a photon of sufficient energy it can result in the emission of an electron and thus the creation of a positively charged ion. Generally, there is little magnetic interaction between the outgoing electron and the nucleus and therefore the energy and direction of the emission of the electron depend only on the electronic configuration of the atoms and the photon characteristics. Therefore, different isotopes of the same atom (same electronic structure: different numbers of neutrons in the nucleus) for atoms with zero spin (see below) give identical electronic dynamics, i.e. same electron energy and angular distributions. However, in many cases the nucleus can have non-zero nuclear spin: this can occur when the neutrons and protons of the nucleus are arranged in such a way as to give rise to a magnetic field. Under certain circumstances, nucleus can exert a force through this field on the electron being emitted and change its angular distribution. The result is that the angular distribution of electrons emitted from atoms with non-zero nuclear spin are more “blurred” than those emitted from zero spin cases where this interaction does not occur.
The experiments, involving scientists from CNR-ISM Istituto di Struttura della Materia in Italy, Skobeltsyn Institute of Nuclear Physics in Russia, European XFEL GmbH in Germany, ISMO-CNRS Université Paris-Sud and SOLEIL Synchrotron in France have been performed at the DESIRS beamline of SOLEIL and have sought to show these effects by measuring the angular distributions of electrons emitted in the two-photon ionization of Xenon atoms. The experiment consisted in first preparing the Xe atoms in well-defined states by exciting the atoms using a single photon of synchrotron radiation. The resulting atoms form an ensemble of excited atoms which “point” at a single direction along the direction of the polarization of the exciting light. A second photon, this time from a visible laser, is used to ionize these atoms and then the angular distribution of the electron emitted is measured. Angular distribution means the probability of emission of the electron in each direction with respect to the direction of polarization of the light. The “trick” of the experiment is always to ionize only one atom at a time so that scientists can keep track of the mass (and therefore the number of protons and neutrons) of the ion formed in coincidence with each electron. Xenon is a good test case as about half of the isotopes have nuclear spin while the other half do not. Using this method one can build up the angular distribution of electrons formed with 129 Xe ions (nuclear spin, I=1/2) and that of electrons formed with 132,134 or 136Xe ions (all zero nuclear spin, I=0). As can be seen in Figure 1 the electron angular distribution is much more “peaked” in the case of the electrons formed with 129 Xe ions than that of electrons ejected from zero nuclear spin atoms. The cause of this is the blurring out of the electrons formed with the non-zero spin nuclei as was touched on above.
A quantitative measurement of these effects allows researchers to understand more clearly the electronic dynamics of the photoionization process thanks to a more straight-forward comparison of the results obtained with theory. Furthermore, these experiments may also give them a way to extract information on the nucleus by measuring the angular distributions of the electrons, i.e. the quantity of blurring is related to the size of the magnetic field created by the nucleus.