How to actively control the diameter of biomimetic nanotubes?
French researchers showed it is possible to generate self-assembling nanotubes which diameter is actively controlled by the environment acidity. Indeed, they used a basic component which exists through two different forms depending on the pH. These two forms spontaneously assemble to create two distinct nanotubes with a diameter of 10 or 50 nm.
Nature excels in the art of forming complex architectures at nanometric scale. For this purpose, it uses the spontaneous self-assembly of “elementary bricks”, similar to LEGO™, which contain the whole information necessary to the establishment of their future contacts within the programmed structure.
This simple process, allowing effortlessly making nano objects in large quantities and with unmatched precision has been an inspiration to scientists. It is indeed possible today to conceive items of any shape and with varied basic functions.
Thus, researchers have discovered a molecule that assembles in aqueous solution to form carbon nanotubes which diameter can be controlled from 11 to 50 nm depending on the pH.
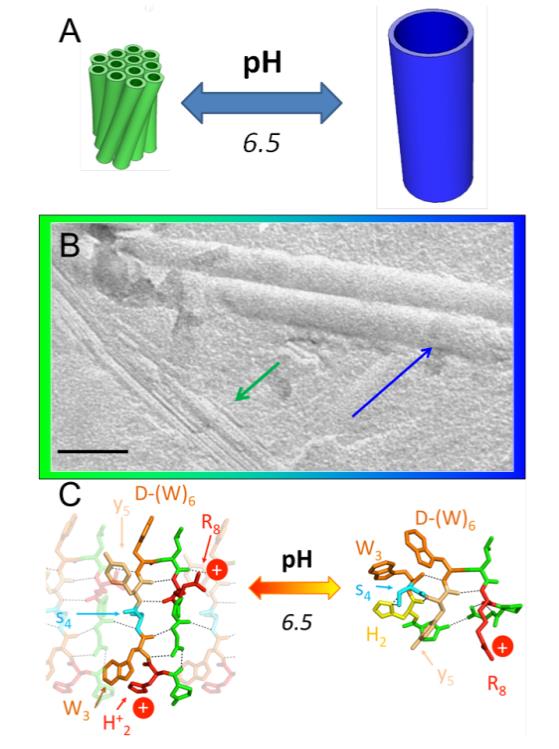
Scientists from CNRS, CEA-iBiTec-S, Rennes 1 University and Ipsen focused on a small molecule called triptorelin. Actually it is a peptide made of 10 amino acids, and an analogue of a natural hormone: gonadorelin. Whenever the drug powder is put into water, a whitish gel appears within a few hours. The texture of this gel surprisingly changes in less than a day depending on the environment acidity. It is even possible de pass several times from one texture to the other. To understand the phenomenon, researchers used the SWING and PROXIMA 1 beamlines at SOLEIL, and quickly suspected the existence of nanotubes with various diameters. Confirmation came from a technique allowing to observe objects at nanometric scale: electronic microscopy (figure1). Researchers needed to know precisely the organization of triptorelin in the various diameters nanotubes to understand the ongoing mechanism. Atomic details could only have been obtained through the combination of several experimental techniques, bringing information at different scales: vibrational spectroscopies, X-ray scattering and diffraction, all available at SOLEIL.
The key of the mechanism relies on a particular amino-acid of triptorelin, a histidine able to fix or not protons depending on the pH. In an acid environment, histidine bounds with a water hydrogen and is thus positively charged. Resulting electrostatic repulsions impose a very specific conformation to the peptide. If the acidity is lowered, the hydrogen is lost and histidine becomes neutral but keeps its affinity for protons and replaces the water proton by another from an amino-acid of the peptide. This mechanism completely upsets its conformation. Geometrical characteristics of self-assemblies obviously depend on the primary conformation of the peptide which is linked to the medium acidity in this case. Nanotubes actively adapt to the change of acidity by modifying their diameter, going from 11 to 50nm.
Precise understanding of the mechanism paves the way for many perspectives. Indeed, the existence of such a “pH responsive” behavior for a peptide involving 7 amino-acids (3 amino acids of triptotein are not involved) allows to think of inserting it easily in other applied systems, in nanomaterials or pharmacology as well.
Moreover, atomic details of the mechanism solved by researchers bring a new light on the conformation changes of virus which also benefit from pH variation.