Exceptional Hydrogen Uptake in Crystalline indium gallium nitride semiconductors: a new strategy for H storage ?
Can semiconductive materials store and deliver a large quantity of hydrogen on demand, like other systems studied for hydrogen storage? Could they be used in hydrogen-fuelled vehicles? To date, large H incorporation has only been reported for amorphous semiconductors. Based on experiments carried out at SOLEIL, researchers from the SIRIUS beamline, National Research Council of Italy and the University of Rome I, estimated an unprecedented hydrogen uptake in a direct gap crystalline semiconductor. Joining H storage and semiconductive capability on a unique platform could open new avenues in energy science.
The irradiation of InN and InxGa1−xN semiconductors with low-energy H ions results in exceptionally high hydrogen uptake in a crystalline semiconductor. This phenomenon is attributed to specific In−H complex formation. By exploiting spectral fingerprints of the In−H complexes observable in In L3-edge X-ray absorption spectroscopy (XAS), the scientists provide direct evidence of complex formation. Density functional theory (DFT) calculations assist in interpreting the XAS spectra and offer insights into the energetics of complex formation. The total amount of reversibly incorporated hydrogen in these semiconductors is quantified and their strengths and weaknesses as innovative materials for hydrogen storage are discussed.
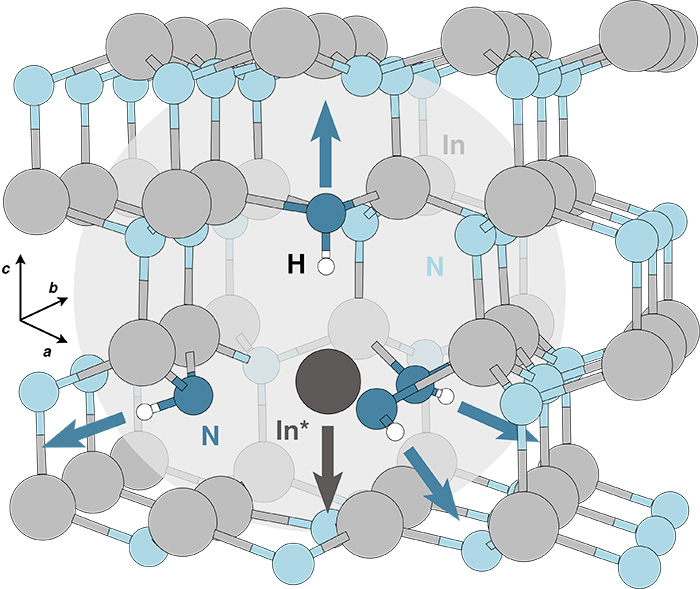
Fig. 1 shows a schematic view of the In* “solitary cation” complex in InN (the structure is similar in InxGa1−xN). Light gray and light blue circles indicate In and N atoms respectively. Darker gray and blue circles indicate In and N atoms involved in the multi-H complex, and white circles indicate H atoms. The arrows indicate the direction of displacement of the complex atoms, with respect to the lattice positions. The light gray circle highlights the In* complex.
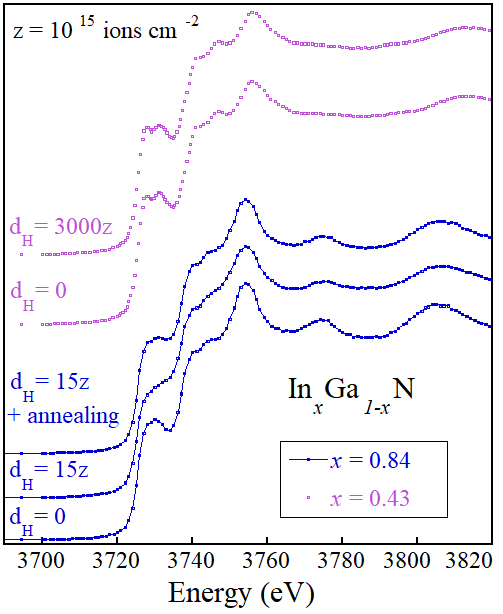
Fig. 2 shows the In L3-edge XAS spectra for two groups of InxGa1−xN epilayers corresponding to the highest (x = 0.84) and the lowest (x = 0.43) In concentration under analysis. For the highest concentration (bottom part of the figure), a hydrogenation at dose dH = 5 × 1016 ions cm−2 is sufficient to induce the transformation of the prepeak at energy = 3729 eV into a shoulder, consequence of the formation of the In−H complex . We also show that the effect on the XAS is reversible since a spectrum taken after a thermal annealing of the hydrogenated sample restores the prepeak and original spectrum line shape (third spectrum from the bottom), as a result of the dissolution of the complex.
A different behaviour is observed in the samples with lowest In concentration (the two upper spectra). In this case, hydrogenation does not induce any modification of the spectrum compared to the as-grown one, despite a very high H dose being used (dH = 3 × 1018 ions cm−2). This observation parallels what was observed for the band gap: the smaller x is, the smaller the shift of the emission peak towards higher energies after hydrogenation, with the effect totally disappearing for x ∼0.49.
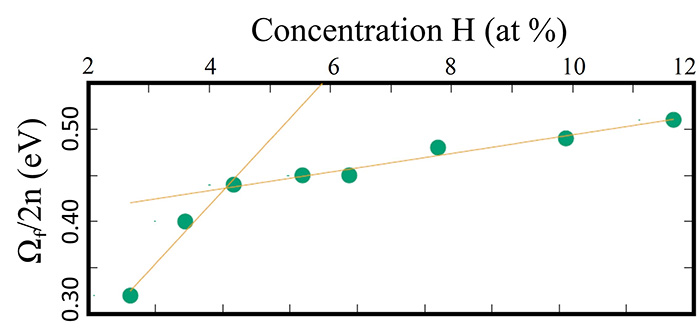
From the XAS spectra and ab initio simulations based on defect configurations calculated by DFT, the maximum concentration of In* complexes in the InN and InxGa1−xN epilayers is estimated. Considering that an In* defect involves four H atoms for each In* cation, the volumetric concentration of H can be extracted, which reaches 27 at % for the InxGa1−xN epilayers hydrogenated at the highest doses. This H incorporation is extremely high for a crystalline semiconductor, which can be explained by the trend of the formation energy as a function of the defect concentration (Fig. 3). DFT predicts two trends, with decreasing slope at higher concentration; this is because of the creation of electronic levels that bandize (merge) after a defect concentration threshold is reached.
Key strengths of these materials include the ability to store H at room temperature and pressure in a reduced volume and within a semiconducting medium. However, challenges remain since the currently high temperature and long time necessary for dehydrogenation pose limitations for automotive applications. Further research and optimization efforts are required to address these challenges and unlock the full potential of this approach for H storage.