Drug translocation across bacterial membrane: New concepts for approaching antibiotic activity
A main challenge in chemotherapy is to determine the in cellulo parameters modulating the drug concentration required for therapeutic action. A method combining spectrofluorimetry and microscopy has been developed by the « Transporteurs Membranaires, Chimiorésistance et Drug-Design » team (Faculté de Médecine et Pharmacie, Aix Marseille University) in collaboration with DISCO beamline to monitor the accumulation of antibotics in Enterobacteriaceae bacteria. Recently, these approaches have been extended for the analysis of individual bacteria.
In Gram-negative antibacterial research, the challenge is to define the antibiotic (ATB) permeation across the membranes. Passing through the membrane barrier to reach the inhibitory concentration inside the bacterium is a pivotal step for antibacterial molecules. Robust methods to visualize and quantify the intrabacterial antibiotic concentration are urgently needed for identifying clear correlations between drug translocation (influx, efflux) and accumulation in both susceptible and resistant strains, and also for clarifying how certain chemical structures can affect drug entry and residence time within the cell.
Optimized spectrofluorimetric and microscopic methods using appropriate calibration curves and internal standards have been developed by scientists from the « Transporteurs Membranaires, Chimiorésistance et Drug-Design » team on the DISCO beamline to monitor the accumulation of fluoroquinolones ATB in Enterobacteriaceae. More recently, these approaches have been extended for the measurement of antibiotic accumulation in intact individual cells.
Tracking and measurement of antibiotics inside bacteria
In order to evaluate the ATB flux through bacteria, several parameters linked to the drug uptake (influx) and expulsion (efflux) must be taken into account. The intracellular concentration of ATB (Cin) and the critical inhibitory intracellular concentration of ATB (Cinh) notably depend on porins and efflux pumps: membrane proteins (pores or “active” transporters) involved in membrane crossing. Moreover, chemical moieties within a specific class of antibiotic class can also have an influence.
The precise determination of both Cin and Cinh, for a given antibacterial molecule for a given bacterial strain, is needed to define the "Resident Time Concentration Close to Target" (RTC2T) concept. RTC2T will be useful to quantify the biological activity of different adjuvants in increasing the intracellular concentration of specific antibiotics in strains exhibiting membrane-associated mechanisms of resistance (influx decreased, efflux increased).
A new concept, "Structure Intracellular Concentration Activity Relationship" (SICAR), also helps defining the ATB profile. SICAR has been elaborated by monitoring real-time accumulation of an ATB inside the bacteria.
Two case studies
The translocation through bacterial membranes via outer membrane pores or transporters has been studied for ATBs belonging to different classes (ß-lactams or fluoroquinolones). Translocation is a relatively fast event and the steady-state concentrations are generally reached after short incubation times. Monitoring real-time accumulation of fluoroquinolones previously showed different accumulation kinetics and steady-state accumulation levels depending on the properties (chemical and structural) of the ATB in a selected bacterial background. Thanks to those results, the SICAR concept ("Structure Intracellular Concentration Activity Relationship") has been elaborated, in order to dissect the profile of antibacterial molecules. SICAR connects the physicochemical drug properties to the efficacy of translocation through the bacterial membrane and the resulting intracellular accumulation.
These parameters were used to dissect the role of chemical structures involved in influx or in efflux, and inform rationale pharmacomodulation of side chains to fine-tune molecular interactions with membrane transporters. Two cases have been studied until now:
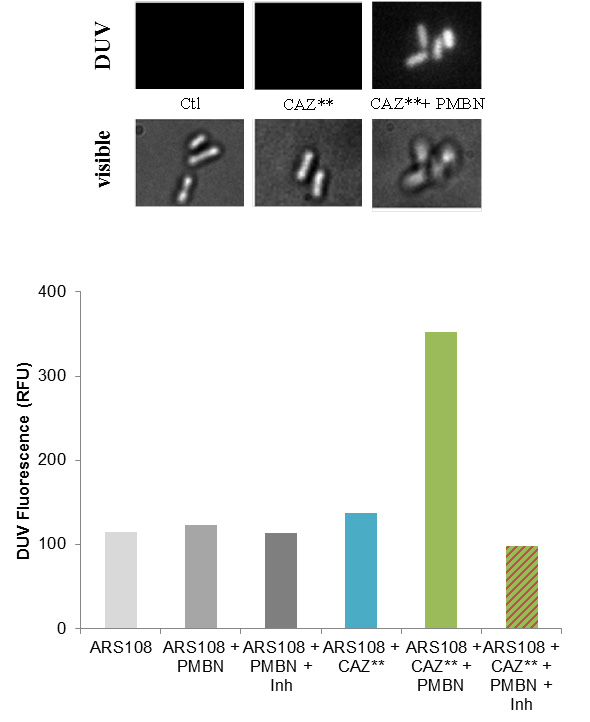
1- Ceftazidime (CAZ), a potent ß-lactam (cephalosporin) used to treat resistant bacteria in combination with ß-lactamase(1) inhibitors. Different fluorescent derivatives were synthesized to dissect the early step of translocation across bacterial membrane by measuring the internal accumulation and activities in clinical resistant strains. By microspectrofluorimetry and epifluorimetry the scientists demonstrated the translocation of CAZ to the periplasmic space when the outer membrane barrier was permeabilized (Figure 1, CAZ** accumulation). This study demonstrated the correlation between periplasmic accumulation and antibiotic activity by approaching ß-lactam permeation relative to membrane permeability.
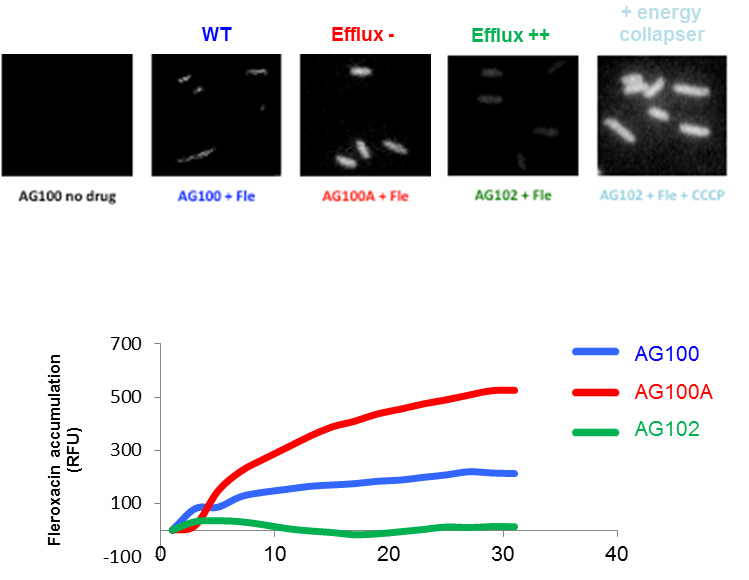
2- A fluoroquinolone has been studied for their accumulation in bacterial population and inside individual Gram-negative bacterial cells that express various levels of efflux pumps (Figure 2). For a given fluoroquinolone library, these assays allowed the determination of SICARIN and SICAREF indexes that reflect “structure-to-penetration” and “structure-to-efflux” relationships, respectively.
RTC2T-SICAR can be used to better understand and improve the antibiotic chemotypes that are related to permeation and efflux. The final objective will be a maximal antibacterial efficacy of the antibiotic against various resistant species.
The research leading to these results was conducted as part of the TRANSLOCATION consortium and has received support from the Innovative Medicines Initiatives Joint Undertaking under Grant Agreement n°115525, resources which are composed of financial contribution from the European Union’s seventh framework program (FP7/2007-2013) and EFPIA companies in kind contribution. This work was also supported by Aix-Marseille Univ. and Service de Santé des Armées, and by SOLEIL programs.
(1) ß-lactamases: bacterial enzymes responsible for the bacterial resistance to the ß-lactams ATB family.