A Complex Trap: Potential new therapeutics for Asthma & common allergies targeting the human TSLP receptor
The pro-inflammatory cytokine1 - thymic stromal lymphopoietin (TSLP) plays a central role in common allergic diseases including asthma and atopic dermatitis. X-ray diffraction data collected at the PROXIMA-2A beamline by scientists from the Flanders Institute for Biotechnology (VIB) and Ghent University in Belgium was used to solve the structures of human TSLP in complex with its receptor, but also with the therapeutic antibody Tezepelumab, which is currently under evaluation as a new treatment for asthma. Complementary biophysical and cellular studies on the cooperative assembly of this key signaling complex enabled the design of novel TSLP inhibitors called TSLP-cytokine traps, which potently reduce the activity of TSLP in vitro and are currently being tested in animal models.
Thymic stromal lymphopoietin (TSLP) is a cell surface receptor from the interleukin-2 (IL-2) cytokine family and is activated in skin and epithelial cells in the lung and gut in response to pathogens. It regulates the immune response at barrier surfaces by driving the activation of immature dendritic cells, mast cells, basophils, eosinophils and lymphocytes.
Aberrant signaling by TSLP has been linked to asthma and a series of allergic diseases and has a potentially massive socioeconomic impact in terms of human healthcare. TSLP is now widely considered to be important in some of the most prevalent inflammatory allergic disorders, such as the atopic diseases (asthma, atopic dermatitis and atopic rhinitis), chronic obstructive pulmonary disease (COPD), and eosinophilic esophagitis. The validity of TSLP as a therapeutic target in humans was demonstrated recently in a clinical trial (Ref) in which asthmatic patients were treated with the anti-TSLP antibody Tezepelumab (AMG-157) developed by Amgen and MedImmune.
To develop therapeutic strategies targeting TSLP, the teams at Ghent University and VIB combined crystallographic, biophysical and cellular studies to obtain structural and mechanistic insights into the assembly of the pro-inflammatory signaling complex, i.e. the TSLP and its receptor, formed by two different proteins: TSLPR and IL-7Rα.
Structure of the human TSLP/receptor complex
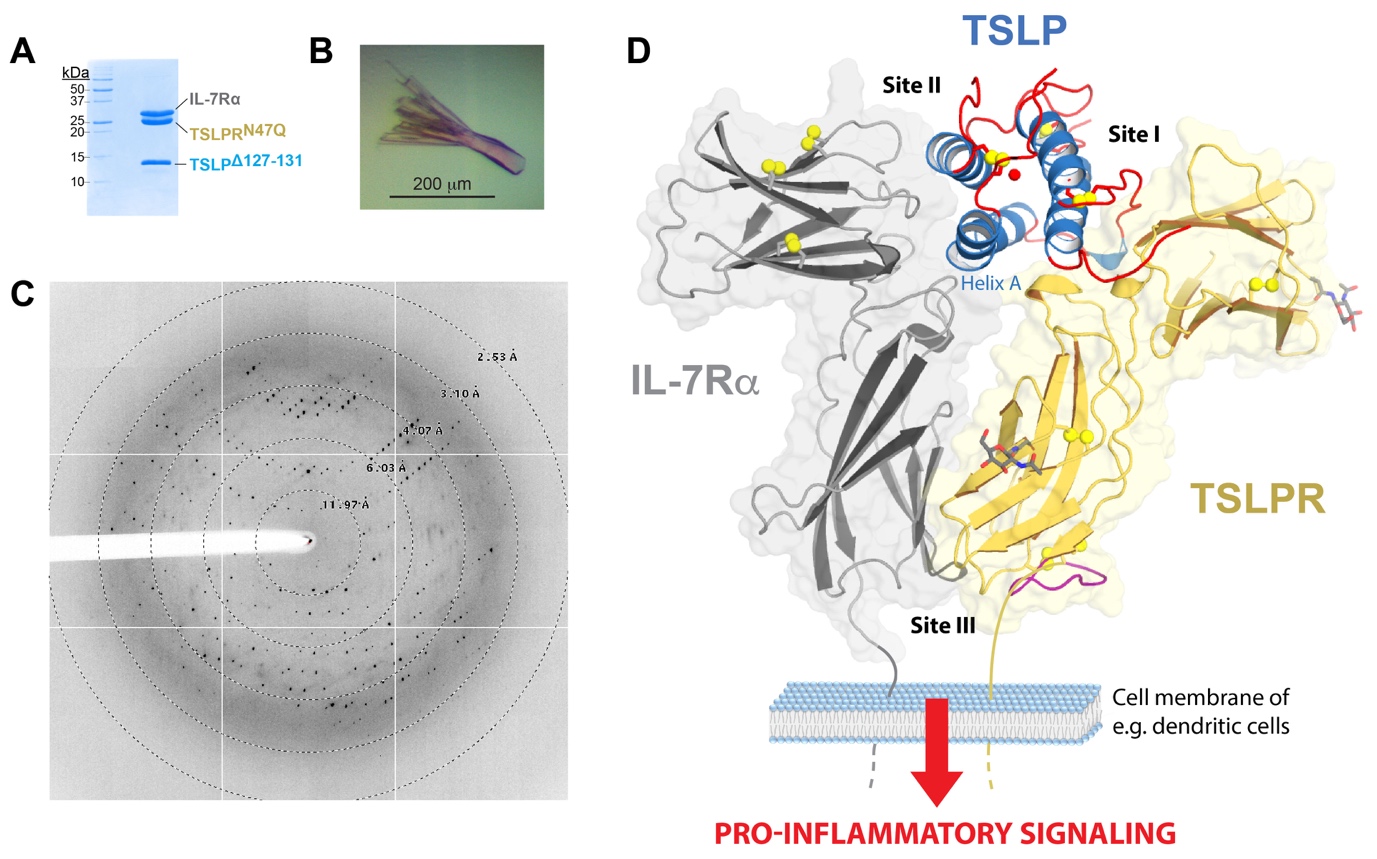
Recombinant proteins were produced in bacteria and glyco-engineered mammalian cells to generate high quality crystals. Optimised crystals of the complex diffracted to 2.55 Å resolution when exposed to the microbeam at PROXIMA2-A (Figure 1A-C), enabling the structure of the human TSLP:TSLPR:IL-7Rα complex to be determined to high resolution.
The crystallographic structure (PDB 5J11; Figure 1D) reveals in detail how TSLP employs two opposing surface patches to interact with the “elbow tips” of the cytokine-binding homology regions of TSLPR (site I) and IL-7Rα (site II). This allows the membrane-proximal parts of the two receptors to engage in heterotypic receptor-receptor interactions (site III).
The structural data – including NMR and MD simulations – combined with binding studies and in vivo assays, led the authors to propose a mechanism where the formation of a TSLP:TSLPR encounter complex is driven by long-range electrostatic attraction of TSLP to its receptor at the cell surface. This primes two concerted structural events: (i) an allosteric activation of TSLP at site II, and (ii) repositioning of the TSLPR membrane-proximal domain to enable interactions with the extracellular domain of IL-7Rα at site III (Figure 1D).
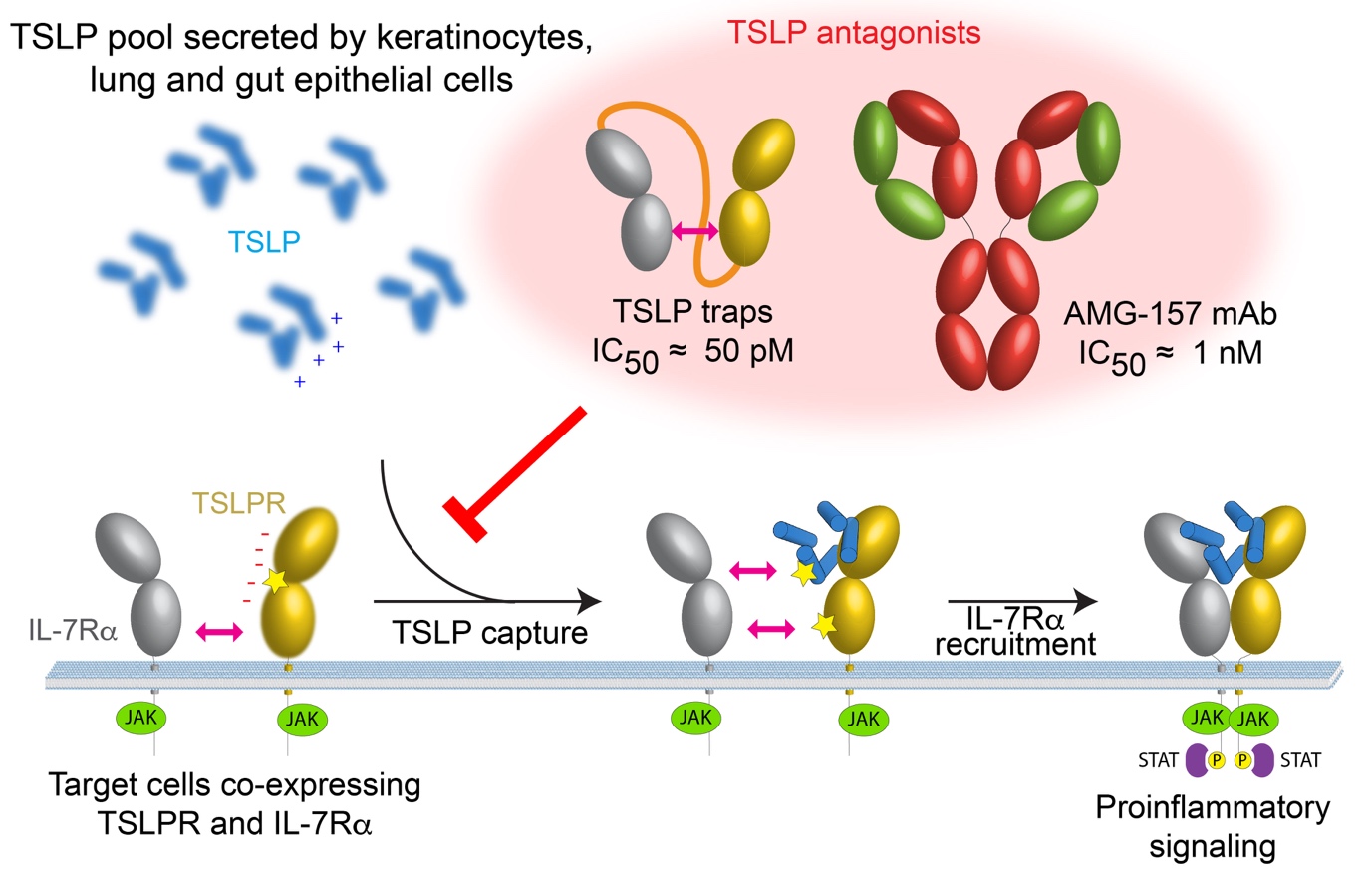
TSLP-traps
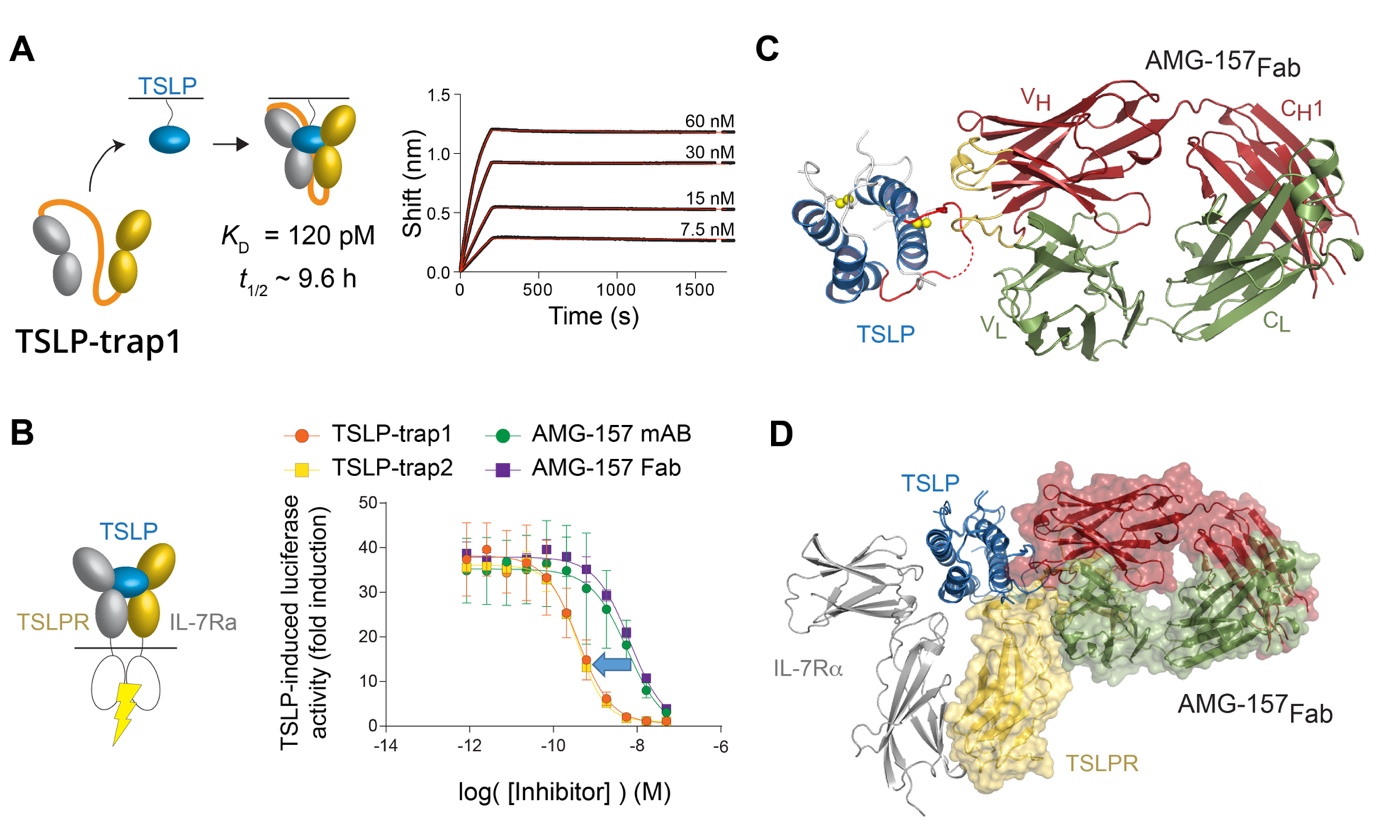
The authors exploited the intrinsic co-operativity of the TSLP:TSLPR:IL-7R complex to develop TSLP-traps, which are highly potent in vitro, by linking the TSLPR and IL-7R ectodomains to create a single protein. TSLP-trap fusion proteins neutralize TSLP by boosting the affinity of complex formation by nearly three orders of magnitude (KD = 120 pM) and drastically reducing the dissociation or off-rate kinetics (Figure 2A-B).
The authors also seized the opportunity to obtain structural and mechanistic insights into how the monoclonal antibody Tezepelumab (AMG-157), currently undergoing trials as an asthma treatment, might exert its antagonistic effects on TSLP. The resulting structure (PDB 5J13, Figure 2C) revealed that AMG-157 inhibits complex formation by binding to, and competing for, the TSLPR binding site of TSLP (Figure 2D).
The mechanism proposed for the assembly of the TSLP signaling complex and possible broader strategies to antagonize pro-inflammatory signaling are recapitulated in Figure 3.
The structural and mechanistic insights, and molecular tools, reported in this study should facilitate a deeper understanding of TSLP signaling in vitro and in animal models, and will guide therapeutic approaches aiming to manipulate human TSLP-mediated signaling to treat allergic diseases.
1 – cytokines : signaling molecules (proteins or glycoproteins)-ex: interferons- produced by cells of the immune system in order to regulate the behavior of cells around them.