A biomembrane model helps to understand the effects of amoxicillin on cells
Amoxicillin (AMX) is an antibiotic whose mechanism of action promotes cell wall lysis and consequently the death of bacteria from several different species. AMX is considered a first-line drug against different bacterial infections, such as against Helicobacter pylori (H. pylori), notably involved in stomach ulcers. However, AMX has been associated with gastrointestinal and renal side effects, with higher toxicity when the pH is lower.
By considering this association and the well-known pH gradient of the gastric mucosa, scientists from UCIBIO/REQUIMTE, I3S, and CESPU (Portugal) wanted to evaluate the influence of pH on the toxicity of AMX, possibility related with a direct cell membrane interaction. Their results, partly obtained on the SIRIUS beamline, are published in BBA, Biomembranes.
Amoxicillin is a semi-synthetic antibiotic used worldwide. It is considered a first-line drug against different bacterial infections and reported as the most often prescribed antibiotic in 22 of the 30 European Union countries.
But AMX is also associated with gastrointestinal and renal side effects, with higher toxicity when the pH is lower. The present study is focused on the study of the impact on the biophysical parameters of the phospholipids present on the mucus layer – whose pH gradient is well-known – after their interaction with AMX.
A biomembrane model and a combination of techniques
For this investigation, monolayers at the air/liquid interface were used as a simplified membrane model, to avoid the interference of trans-bilayer events and to maintain the properties of biomembranes. Both the phospholipid composition and the pH were selected according to the aim of the study. For that purpose, 1,2-dipalmitoyl-sn-glycero-3-phosphocholine (DPPC) monolayers were used since phosphatidylcholines are the most common phospholipid headgroup of biological membranes. To have insight of the effects of AMX, different techniques were employed, namely, thermodynamical measurements, infrared reflection-absorption spectroscopy (IRRAS), synchrotron grazing incident X-ray diffraction (GIXD) on the SIRIUS beamline, and Brewster angle microscopy (BAM).
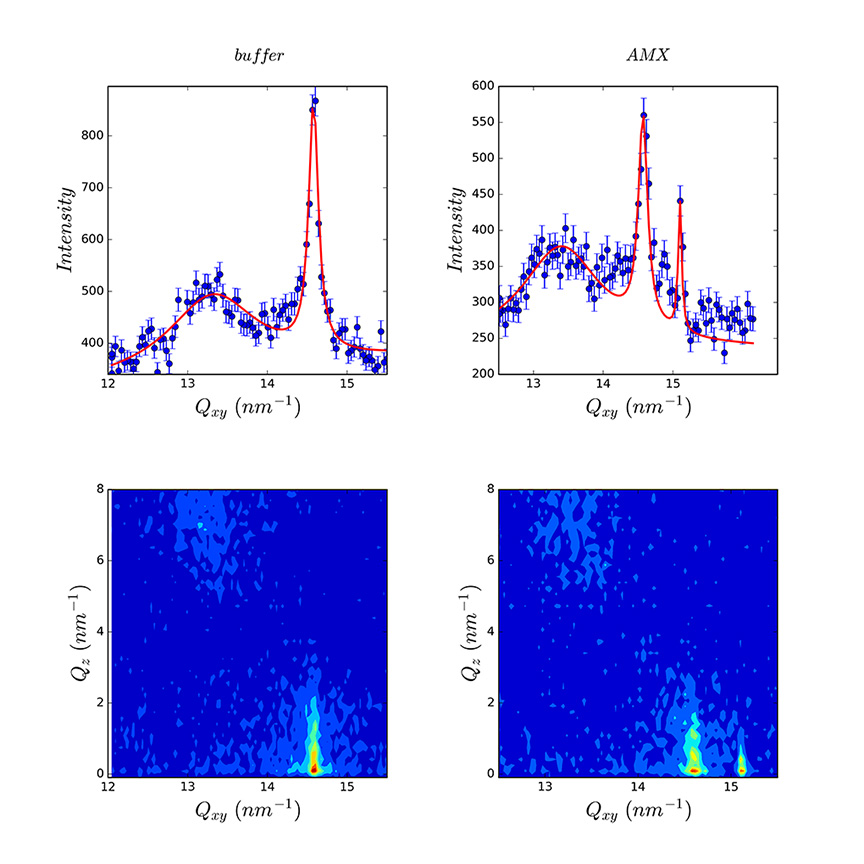
The monolayers of DPPC spread onto different buffer solutions (pH 1.2, pH 5 and pH 7.4) showed different structural and packing properties for which GIXD experiments are useful.
The interaction between AMX and a DPPC monolayer depends on the protonation state of both AMX and DPPC, which changes depending on the pH.
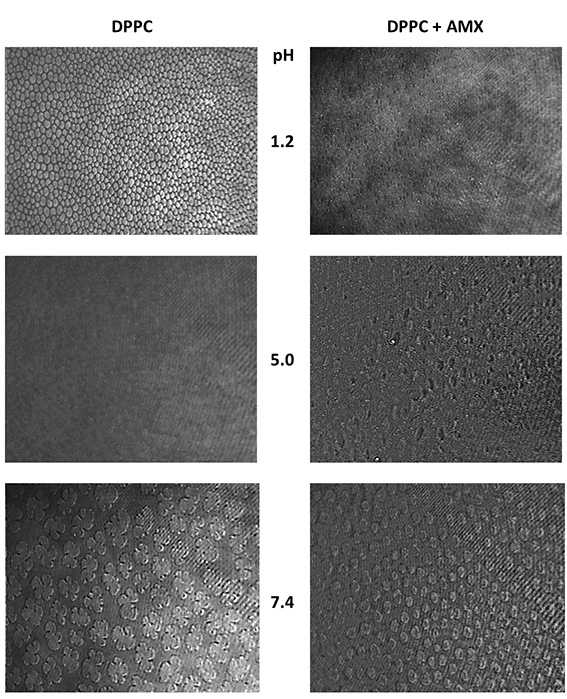
At pH 1.2, the presence of AMX leads to a highly packed monolayer. The GIXD results showed a higher effect at lower pressures, with alteration of the tilt angle of the hydrophobic chains. From the BAM images, the formation of pores is visualized in presence of an AMX subphase (figure 1).
At pH 5, the overall effect of AMX is similar to the one observed at pH 1.2. Macroscopically (Langmuir surface pressure – area isotherm), the changes in the area per lipid molecule and in the elastic modulus are coherent with a higher packing of the phospholipids in presence of AMX.
This effect is also reflected in the PM-IRRAS results and in the BAM results, in which at 15 mN.m-1 a lipid film is visualized in presence of AMX, whereas separated domains are seen in plain DPPC.
The presence of pores is also visualized even though the protonation states of both DPPC and AMX are different from the ones verified at pH 1.2.
The changes in the structural arrangement of DPPC are reflected in the additional Bragg peak in the presence of AMX (figure 2). It suggests the demixion of the film in pure DPPC domains and DPPC domains affected by the presence of AMX.
At pH 7.4 and at 10 mN/m, the interaction between AMX and DPPC may be established due to the reduced packing. By considering the GIXD results and the overall effect of AMX, the scientists suggest that at 10 mN/m electrostatic interactions are established between the negative charge of AMX and the positive charge of the choline of DPPC. Thus, the rest of the molecule may be intercalated among phospholipid chains, which prevents the tilt of DPPC phospholipids and changes its structural arrangement. According to the Langmuir isotherm, AMX also enhances the fluidity of the lipid film. However, at higher pressures, AMX seems to be squeezed out from the lipid film, as suugested by the Langmuir isotherm. The results from the BAM technique are also coherent with this interpretation, since at 10 mN/m AMX changes domains shape, having however no visual effect at higher pressures.
Changes in the pH affect the protonation state of both AMX and DPPC and, consequently, their interaction.
AMX is able to induce the formation of pores in the lipid film of DPPC at both pH 1.2 and pH 5, possibly due to dimerization of AMX under acidic conditions. The disturbance of biological barriers at acidic conditions and, ultimately, the formation of pores may be related with the gastric in vivo toxicity of AMX.
At pH 7.4 and low surface pressure, AMX decreases the organization of the monolayer. However, at higher pressures, namely at 30 mN/m (the lateral pressure of biological membranes), AMX is expelled from the lipid film, with partial restoration of the biophysical properties of the monolayer.
At acidic pH, a higher perturbation is shown, in which pores were visualized by Brewster angle microscopy. These perturbations may ultimately be related with amoxicillin toxicity.
Thanks to a combination of techniques, including GIXD on the SIRIUS beamline, this work demonstrates that changes in the pH affect the protonation state of both AMX and DPPC and, consequently, their interaction.
Ultimately, this may affect the toxic effects of AMX observed in vivo.