Atomic exploration of calcareous precipitates inside bacteria
Some bacteria form micrometric calcareous granules inside their cells, thus storing calcium and carbon. Their function remains unknown but cells spend energy to synthesize them. To predict the chemical behavior of these granules, we need to determine how the calcium and carbon atoms are arranged in space. The use of the LUCIA beamline showed that the calcium in the granules represents a large part of the calcium in the cells and that the arrangement of the atoms made the granules modular reservoirs that can store but also efficiently release the chemical elements composing them.
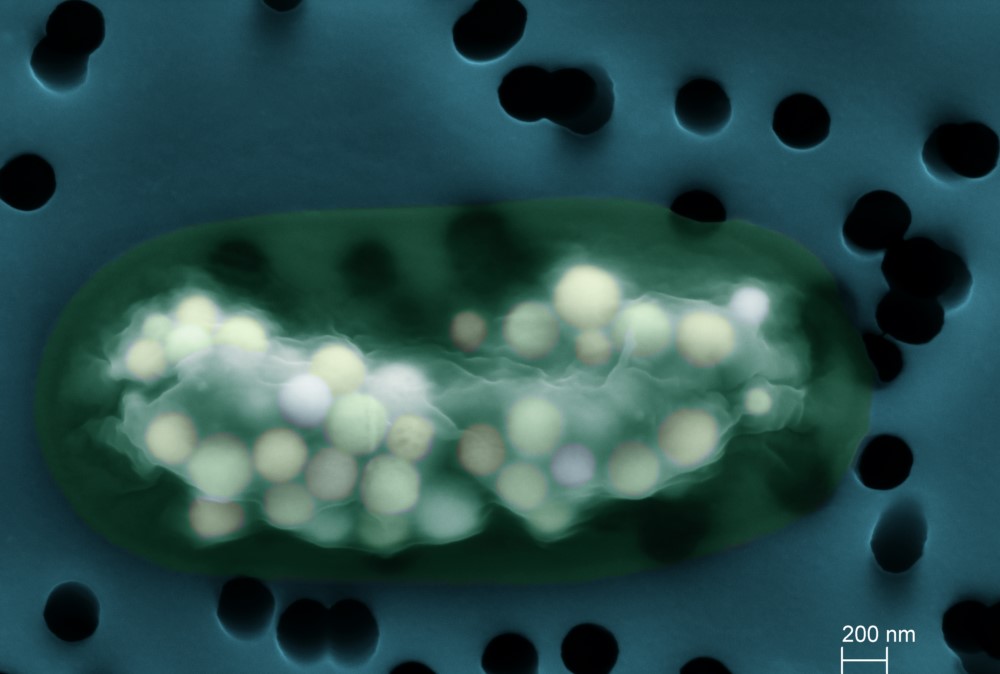
Cyanobacteria are bacteria carrying out oxygenic photosynthesis, a metabolism using light to transform CO2 into organic matter and oxidize water into oxygen. Appearing more than 2.3 billion years ago, they have had a considerable impact on the chemical functioning of the Earth. First, they have oxygenated the atmosphere (there is 20% O2 in the atmosphere today compared to 0% 3 billion years ago). Second, by trapping CO2, they promote the precipitation of carbonate minerals (e.g., CaCO3), forming rocks preserved in the geological record. For a long time, it was thought that this cyanobacterial biomineralization only occurred outside the cells and was a secondary product of their metabolism. However, scientists found that many species formed carbonate phases intracellularly, suggesting much stronger biological control. The latter are diverse, abundant in certain environments and it is a very old process, raising questions about its geochemical impact. To better understand how these mineral phases are distinguished from extracellular carbonates, a collaboration was established between members of IMPMC (Paris), ISTerre (Grenoble) and the LUCIA beamline of the synchrotron SOLEIL.
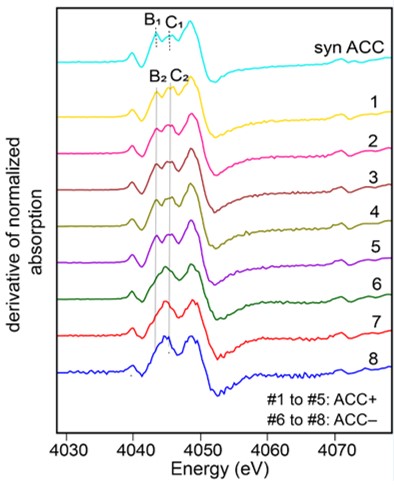
Using XANES spectroscopy at the calcium K-edge on the LUCIA beamline, the scientists analyzed strains of cyanobacteria forming intracellular CaCO3, others not, and different (polymorphic) forms of CaCO3, crystallized or amorphous, synthesized in the laboratory. The spectra obtained show characteristic signatures of the different phases and demonstrate that cyanobacterial intracellular calcium carbonates are amorphous (Amorphous Calcium Carbonate acronymized as ACC), meaning that their atoms are not regularly arranged in a medium- to long-range order. Analyses show that the Ca stored within ACCs is in the majority, constituting between 60 and 84% of total cellular Ca, with the remainder corresponding to Ca complexed by organic molecules and Ca co-precipitated in polyphosphates.
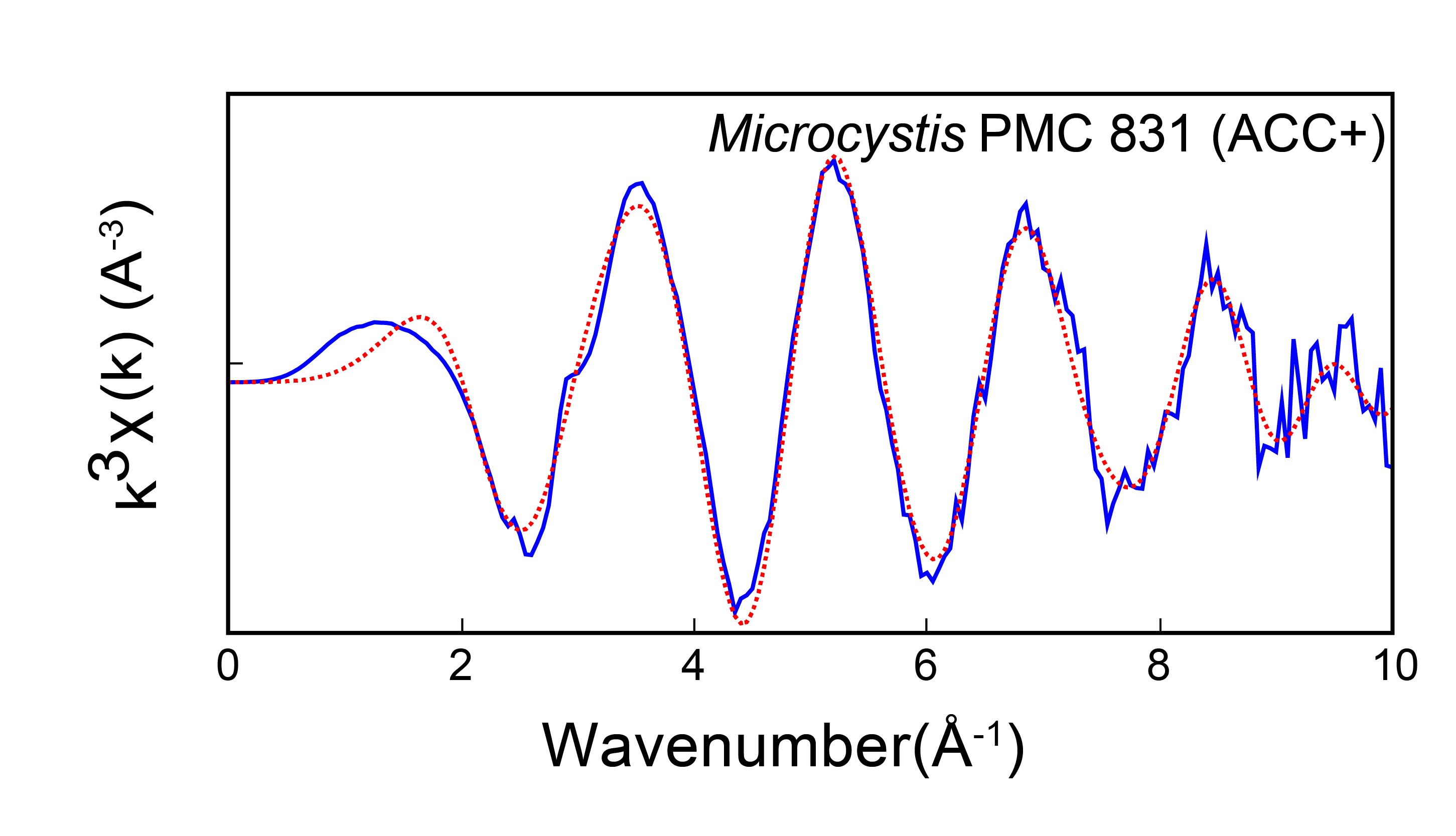
On the LUCIA beamline, they also measured EXAFS spectra of cyanobacteria forming intracellular ACC. Modeling of the spectra allowed to determine the local structure around Ca in these ACC. Comparison with previously studied carbonate structures suggested two things: (i) cyanobacterial ACC has a similar structure but with some differences compared with ACC formed by other biological organisms; (ii) although cyanobacterial ACC is amorphous on a large scale, it has a defined local structure, close to that of monohydrocalcite, a crystalline calcium carbonate with a structural water molecule. This suggests that cyanobacterial ACC could be hydrated. Interestingly, hydrated ACCs are more stable, i.e. they do not crystallize spontaneously, which could explain the observed stability of cyanobacterial intracellular ACC.
To conclude, cyanobacterial CaCO3 inclusions (calcium carbonate forming and persisting inside cells) are amorphous but show a define local structure on the scale of a few atoms. These carbonates are probably hydrated, which limits their spontaneous crystallization. Thus, these inclusions have good properties to store large quantities of chemical elements in cells, remaining easily remobilizable due to their high reactivity and they do not spontaneously crystallize which would be to the detriment of cellular structures integrity.
In the future, the scientists would like to determine the fate of these inclusions when cells die. Can they, upon contact with extracellular solutions, transform into stable crystalline solids, traces of which can be found in the geological archives?