2D magnetic coupling between Mn centers in a metal-organic network
Organic molecules can be intercalated by magnetic atoms on a surface producing two-dimensional metal-organic networks. Whether such systems can display magnetism was the subject of a study performed recently at SOLEIL by a joint team from France (IM2NP, CNRS/ Aix-Marseille University) and United Kingdom (Diamond Light Source). Experiments were performed at the DEIMOS beamline where atomic microscopy can be coupled with magnetic measurements in extreme conditions.
Functional organic molecules are able to manipulate the electron spins of host metal atoms in a controlled way. They can also act as bridges between magnetic atoms, serving as linkers of an ordered lattice. The magnetic properties of the whole system will depend on several parameters such as the lattice spacing and shape as well as the metal-organic bonding. Nowadays it is well known that a fruitful way to control such parameters is to exploit the self-assembly properties of organic molecules in 2D and to build the metal-organic networks using a surface as a support. In practice this is done by co-depositing specifically designed organic molecules and magnetic atoms on a freshly prepared, atomically clean surface.
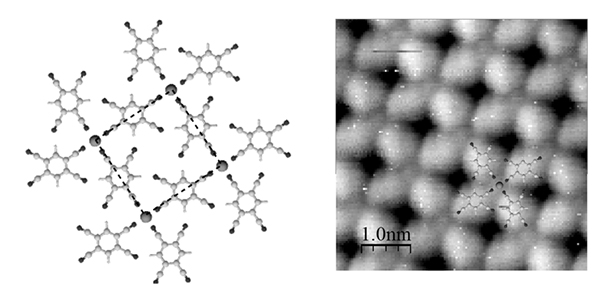
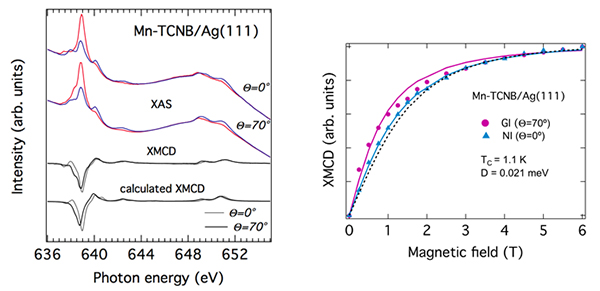
In the present study the preferential bonding between magnetic Mn adatoms and periphery groups of tetra-cyano-benzene (TCNB) molecules were used to create extended domains of Mn-TCNB networks on silver and gold substrates (Fig. 1-a). These were characterized by STM (Fig. 1-b) in a dedicated apparatus connected to the end station. The further magnetic characterization made use of the chemical and magnetic sensitivity of synchrotron circularly-polarized soft X-rays. Namely, by cooling the sample to extremely low temperatures, the magnetic response of Mn centers in the Mn-TCNB network was measured by X-ray magnetic circular dichroism (XMCD) in different geometries (Fig. 2-a). A theoretical modeling of the spectra allowed extracting important parameters such as the anisotropy energy of the Mn atoms suggesting an easy plane of magnetization within the surface. A further investigation was performed by repeating the measurements with different values of the magnetic field. The resulting magnetization curves displayed in Fig. 2-b clearly deviate from the paramagnetic behaviour (Brillouin function) expected in the absence of interaction between Mn atoms. This reveals the presence of a (anisotropic) low temperature Mn-Mn ferromagnetic coupling. To go further, scientists are planning to modify the magnetic coupling by inserting the magnetic centers into an extended covalent network. Transport of magnetic information should thus be favored.