ROCK

ROCK : Rocking Optics for Chemical Kinetics
ROCK time-resolved X-ray absorption spectroscopy (XAS) beamline Energy range 4 - 40 keV
This acknowledgement is mandatory for all publications from ROCK beamline :
This work was supported by a public grant overseen by the French National Research Agency (ANR) as part of the “Investissements d’Avenir” program (reference : ANR-10-EQPX-45)
Major publications in connection with the beamline :
La Fontaine C., Belin S., Barthe L., Roudenko O., and Briois V. "ROCK : A Beamline Tailored for Catalysis and Energy-Related Materials from ms Time Resolution to µm Spatial Resolution" Synchrotron Radiation News, 33(1):20-25. (2020).
Briois V., La Fontaine C., Belin S., Barthe L., Moreno T., Pinty V., Carcy A., Giradot R. and Fonda E. "ROCK : the new Quick-EXAFS beamline at SOLEIL" Journal of Physics Conferences Series, 712 : art n° 012149 (2016).
The purpose of this EQUIPEX project is to build within the existing SOLEIL synchrotron facility and to operate for the benefit of academic and private research projects a new time-resolved X-ray absorption spectroscopy (XAS) beamline based on the quick-scanning energy principle, the so called QuEXAFS.

The ROCK beamline (ROCK being the acronym for Rocking Optics for Chemical Kinetics) is devoted to the study of fast kinetic processes in nanomaterials used in catalysis and batteries. The objective is to contribute to the development of more efficient catalysts and batteries which should find successful industrial applications in the field of energy generation and storage in compliance with the protection of public health and environment. The better knowledge at the atomic scale of nanomaterials involved in catalysis or energy storage provided by time-resolved XAS is recognized by the concerned communities as mandatory for establishing synthesis strategies leading to important breakthroughs in the production of energy from renewable sources and in the development of advanced energy storage devices.
Beamline news
All Rock's news
SOLEIL Highlights 2024
Portrait of Stéphanie Belin - "Understanding how materials work and uncovering their (...)
Team
* External service provider, temporary or collaborator
Collaborator
IADECOLA Antonella (RS2E CNRS)![]() 01 69 35 94 68
01 69 35 94 68![]() antonella.iadecola@synchrotron-soleil.fr
antonella.iadecola@synchrotron-soleil.fr
Technical data
- Energy range
-
from 4.5 to 40 keV
- Energy resolution (∆E/E)
-
2 10-4 to 5 10-5
- Source
-
bending magnet (Ec = 8.65 keV)
- Flux
-
Si(111) @ 8.0 keV : 2 1012ph/s
Si(220) @ 20 keV : 5 1011ph/s
- Optics
-
Collimating toroidal mirror of Si with a coating of Ir (50nm) ;
2 QuEXAFS (Si111 and Si220) monochromators between a flat mirror and a collimating mirror both in Si with three stripes (Pd, Pt and B4C) for harmonic rejection
- Sample environment
-
Catalysis devices (ovens, gas rack distribution, mass spectrometer ...)
Electrochimical thermostable cells multichannel potentiostat
Raman and UV-visible spectrometers
Differential Scanning Calorimetry
- Beam size
-
horizontal : from 350 µm to 4.9 mm (FWHM)
vertical : from 190 µm to 2.2 mm (FWHM)
- Detectors
-
OKEN ionization chambers
Avalanche photodiode
Scientific opportunities
| Nanomaterials | used in catalysis and batteries |
|---|
More information : on the optics (mirrors and monochromators) of the optical hutch, and on the description of the EXAFS experimental hutch.
Optical Hutch
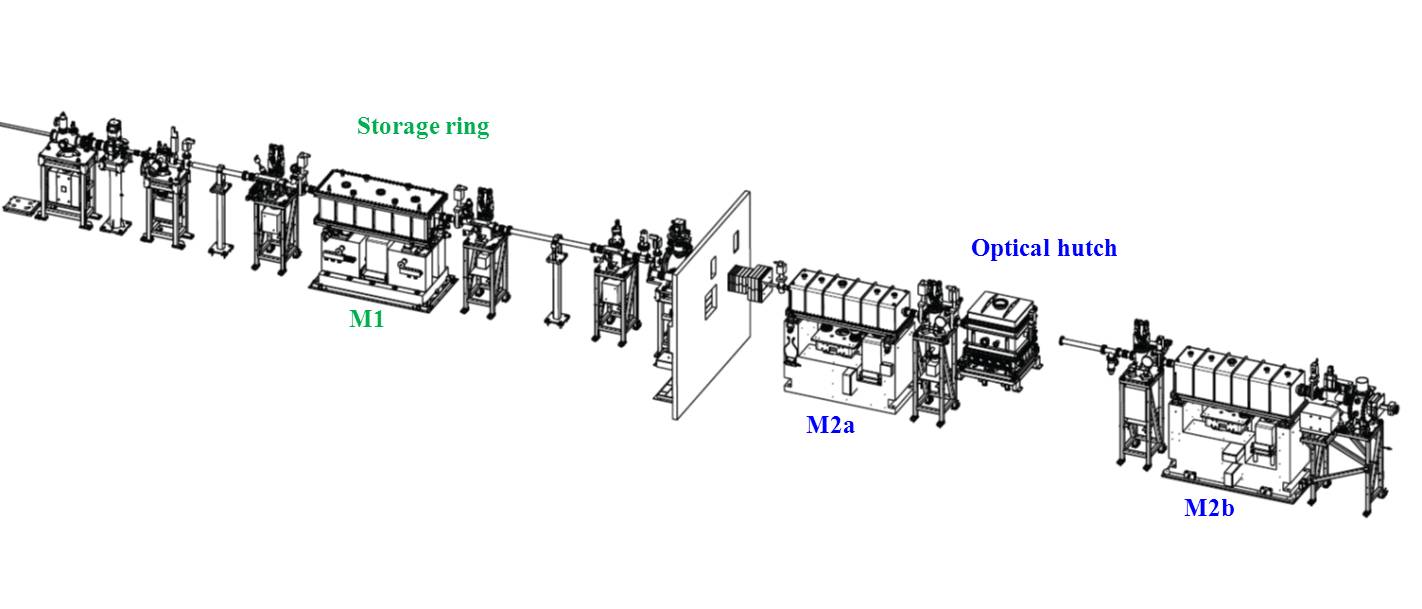
Storage ring : Toroidal collimating mirror M1 (CINEL/Winlight X)


Fixed Grazing Incidence : 2.5 mrad
Ir (50 nm)
Roughness 5 Å <
Tangential slope error: 0.5 μrad RMS
Sagittal slope error : 20 μrad RMS
Water Cooled
Total absorbed power : 87 W
Absorbed power density : 4 mW/mm 2
Collimating principe :
Optical hutch : Mirrors M2a and M2b harmonic rejection and vertical focusing (IRELECALCEN/Winlight X)
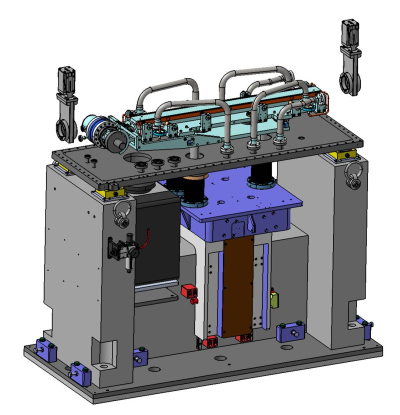
M2a | 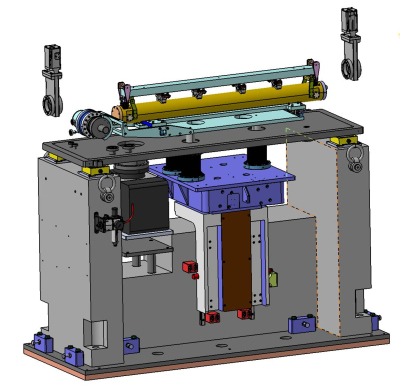
M2b | |
| Acceptance | 1.5 (H) x 1.0 (V) mrad2 | 1.5 (H) x 1.0 (V) mrad2 |
| Source distance | 16.82 m | 22.44 m |
| Optical active area | 47 mm x 1100 mm | 47 mm x 1100 mm |
| Coating | 3 stripes Pt layer 50 nm, Pd layer 50 nm and B4C layer 5 nm | 3 stripes Pt layer 50 nm, Pd layer 50 nm and B4C layer 5 nm |
| Roughness | less than 3 Ǻ RMS | less than 3 Ǻ RMS |
| Longitudinal slope error | 1 µrad RMS/best spherical fit | 1 µrad RMS/best spherical fit |
| Sagittal slope error | 5 µrad RMS | 5 µrad RMS |
| Orientation | Reflecting vertically upward | Reflecting vertically qownward |
| Working pitch angle | 1.75 mrad to 5.2 mrad | 1.75 mrad to 5.2 mrad |
| Shape | Flat mirror : R > 20 km | Bending radius range : 1.5 km < R < 20 km |
| Cooling system | Water cooling | No cooling |
| Maximum total absorbed power | 78 W | - |
| Maximum absorbed power density | 19 mW/mm2 | - |
Quick-EXAFS monochromator
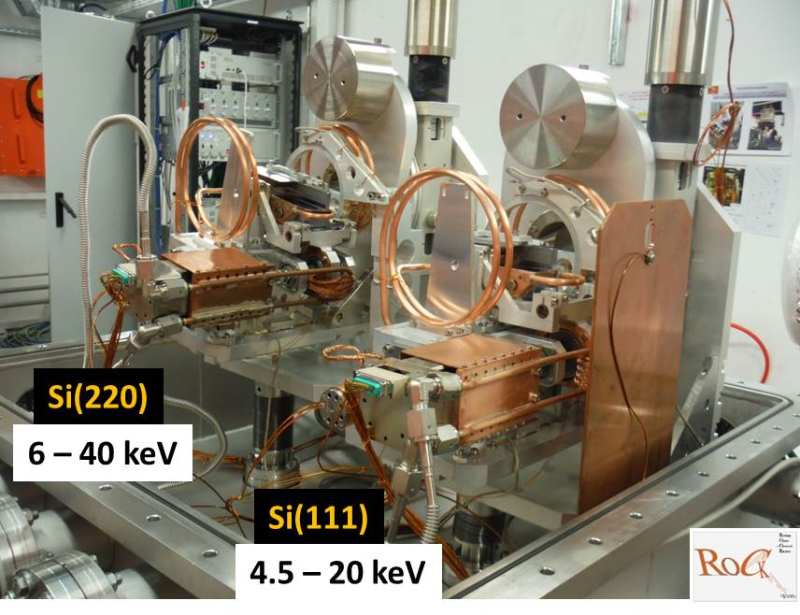
The SOLEIL designed Quick-EXAFS monochromators are made of two independent channel-cut crystals (Si(111) and Si(220)) and was first used on SAMBA beamline. Each channel-cut crystal is mounted on a cam driven tilt table allowing it to oscillate periodically with amplitude θ(t) around an average fixed Bragg angle θB. The amplitude can be tune from 0.1 to 4° by an original SOLEIL in vacuum variable cam design. The θB Bragg angle is selected by one of the two goniometers holding the oscillating tilt tables. The 4.5 to 40 keV energy range is available using both Quick-EXAFS monochromators. The channel-cut crystal can be changed by the other one in few seconds during an experiment allowing to record quick-EXAFS data at absorption edges very far in energy. For instance the XAS study of a bimetallic catalyst at both edges during the same catalytic reaction is now possible with the ROCK’s QuEXAFS set-up, even if the edge energies of those elements are covered by two distinct crystals.
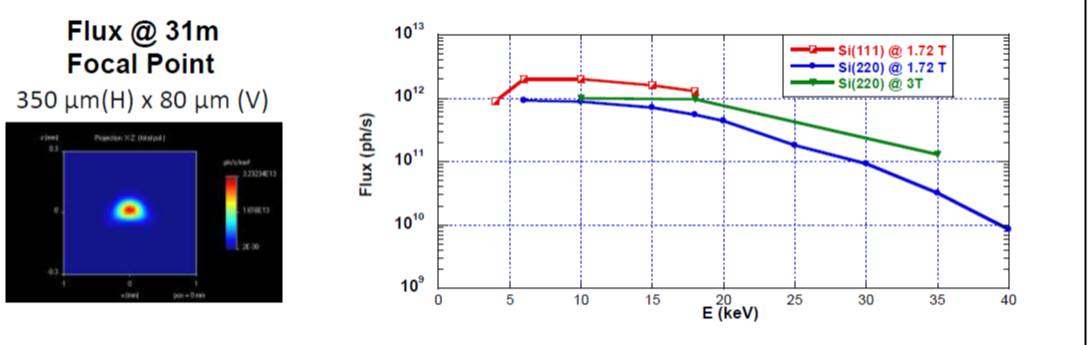
Maximum performance of the Quick-EXAFS mechanics in terms of oscillation frequency of the channel-cut is 40 Hz for two recorded spectra: one with the Bragg angle collection in the downward direction and the other with the Bragg angle collection in the upward direction. Due to the photon flux available at the sample position at the SAMBA beamline in QuEXAFS configuration ((flux ~ 1011 ph/s at 8 keV) the optimal time resolution of experiments on real concentrated and not too absorbing samples is around 100 ms. On ROCK we expect to reach 1012 ph/s in 50 ms.
Related publications:
The SAMBA Quick-EXAFS monochromator: XAS with edge jumping
E. Fonda, A. Rochet, M. Ribbens, S. Belin, L. Barthe, V. Briois
Journal of Synchrotron Radiation (2012), 19, p 417 - 424
EXAFS Hutch
►Quick-exafs Mode
Crystal type : Si111 Si220 (4 to 40 keV)
Beam size : from 350 x 79 µm2 to 4.9 x 2.2 mm2
Flux ~ 1012 ph/s at 8 keV
Transmission

Fluorescence
TEY
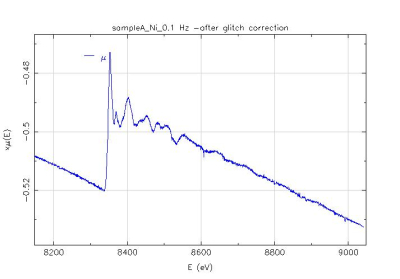
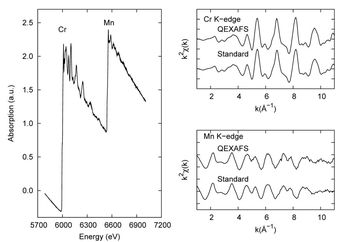
Ni K edge XAS signal (up) collected in a TEY detection mode on a Proton Exchange Membrane Fuel Cell thick film in the Quick-EXAFS mode after correction of glitches. The presented spectrum is not averaged (5s of time acquisition).The frequency of oscillation of the Si(111) channel-cut was 0.1 Hz (5s per spectrum) with an oscillation amplitude of 1.6°.
No picture. The sample must be electrical conductor otherwise a thin layer of graphite may be evaporated.
Edge Jump
Large energie range from 4 keV (Si111) to 37 keV (Si311) in 30s
The remote selection of the monochromator crystals, Si(111) or Si(311), allowing users to permanently access energies between 4 and 37 keV in Quick-exafs mode.
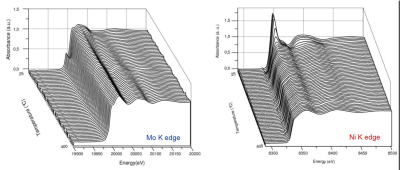
Example of the energy range accessible with Si111 cam=4°
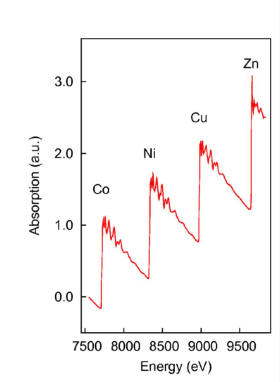
Set-up and combined experiments
CombinedTechniques
ROCK's experimental station EXAFS is characterised by a lot of techniques combined to XAS, such as Differential Scanning Calorimetry, Raman Spectroscopy and UV-Visible Spectroscopy.
XAS with Raman spectroscopy 
| Laser excitation : 785 nm or 532 nm CCD: 100-3450cm-1 or 200-4000 cm-1 Optical fibers with probehead Several Long Working Distance objectives (150 mm to 35 mm) |
XAS with UV-visible spectroscopy 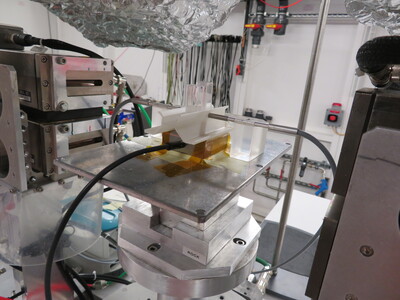
| With optical fibers 190 < I < 1100 nm VScan < 24000 nm/min XAS cell with mylar or PP windows for optical fiber used in transmission or immersion probe : 2 or 10 mm or cuvettes Quartz, Glass, PMMA, PS |
XAS with Raman and UV-Visible spectroscopies 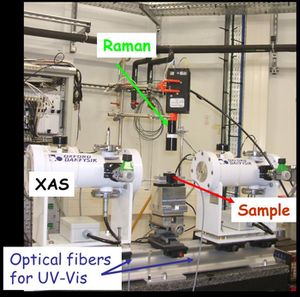
| |
XAS with Differential Scanning Calorimeter (DSC) 
-120 to 800°C | DSC 111 Setaram : * Phase transition (fusion, crystallization, glass transition, polymerization, degradation) |
Catalysis
Retrouvez ci-dessous les différents environnements échantillons catalyseurs à votre disposition sur la station EXAFS.
Polyvalent SOLEIL development for fluorescence, transmission and Raman coupling La Fontaine, C., Barthe, L., Rochet, A., & Briois, V. | 
Two ovens/capillaries set-up collaboration MAX IV/SOLEIL designed by L. Barthe 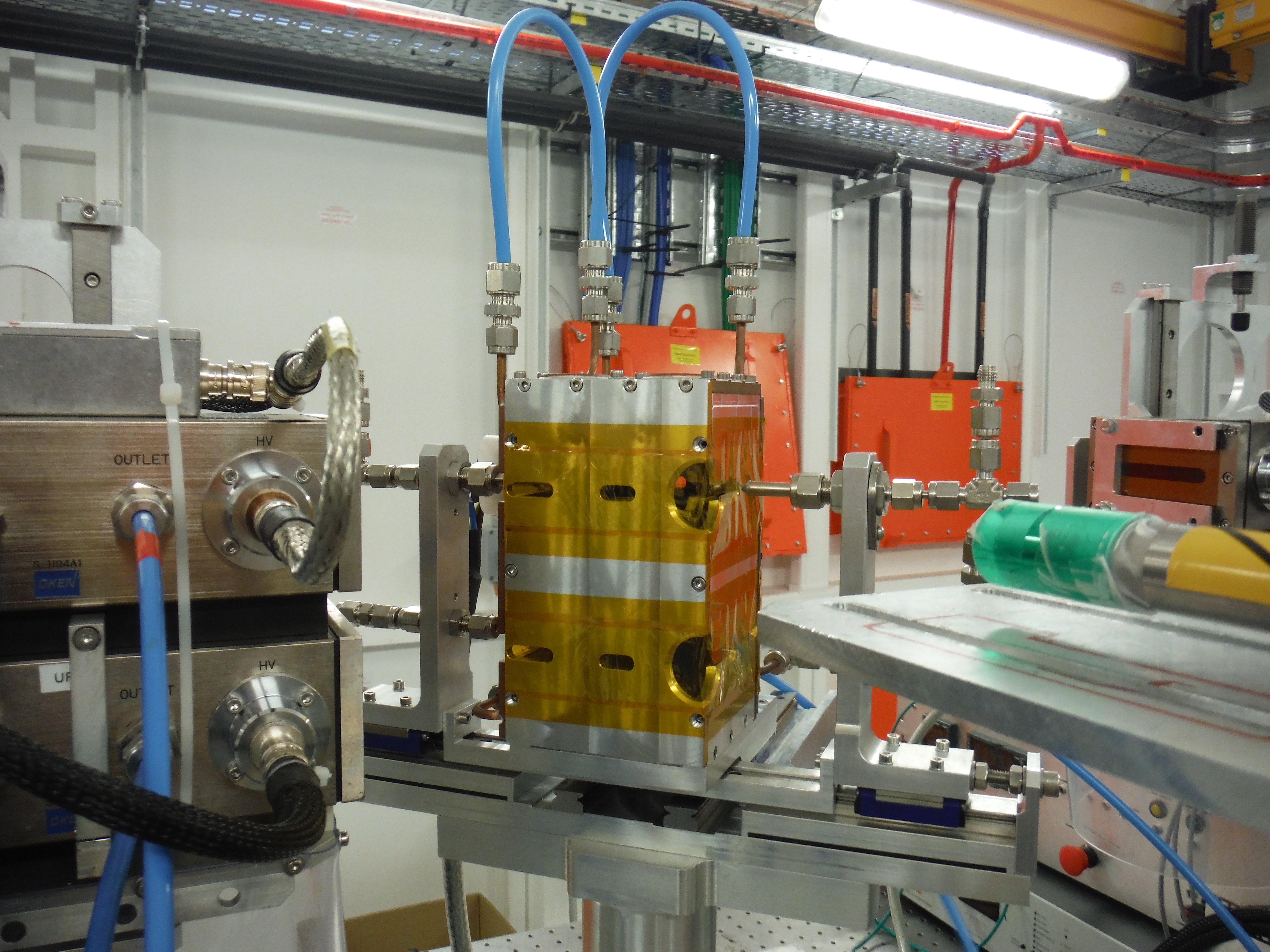
| Characteristics Lytle-type cell
Characteristics 2 capillaries oven
|
High pressure cell for transmission and Raman coupling A. Rochet et al. Catalysis Today (2011) vol 171 186-191 | 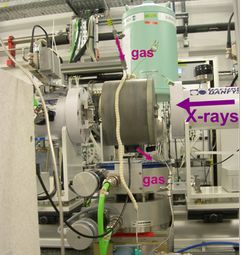
| Characteristics Lytle-type cell

|
Commercial cell Harrick for fluorescence and Raman coupling C. La Fontaine et al. Harrick Scientific Products (Ed.), Application Note n°91201 (2010). | 
| Characteristics
|
| Gas distribution system | 

|
|
| Mass spectrometer | 
| MKS Cirrus
|
Low temperature
With the courtesy of SAMBA beamline
| Helium Cryostat | 
| He cryostat suitable for transmission and fluorescence detection (20 K to Room Temperature) |
| Nitrogen Cryostat | 
| Liquid nitrogen (80 K) cryostat suitable for transmission and fluorescence detection |
Liquid Cells
We have different cells with fixed or variable optical path available for basic or acidic solution. Two of them are thermostatically controlled. Note Kapton, Mylar, PP or PTFE can be used as windows depending of the nature of the solution.
| Liquid cell with variable optical path for transmission | 
| Thermostated liquid cell (PTFE) with adjustable optical paths (10 µm to 6 mm) suitable for the combination of transmission XAS with UV-Vis spectroscopies (design SPECA) |
| Liquid cell for transmission | 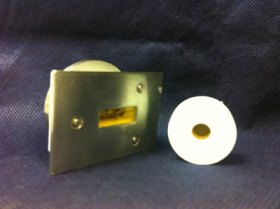
| Liquid cells PTFE with fixed optical paths (0.5<o.p.<5mm) |
| Liquid cell for fluorescence mode | 
| Liquid cell (PCTFE) for fluorescence detection (design F. Villain) |
| Liquid cell for biological sample | 
| PTFE sample-holder for He cryostat With the courtesy of SAMBA beamline |
| Thermostated liquid cell | 

| Thermostated liquid cell (PCTFE) with adjustable optical paths (100µm to 10mm) suitable for the combination of transmission XAS with Raman and/or UV-Vis spectroscopies
Thermostated liquid cell (PTFE) with adjustable optical paths (10µm to 6mm) suitable for the combination of transmission XAS with UV-Vis spectroscopies (design SPECA) |
Energy Science
Setup for Li/Na batteries

| VMP3 Bio-logic Multi-channel Potentiostat 16 independent channels, 6 already equipped (+/-400mA) whose one for impedance measurement |

ANR | Electrochemical cell dedicated to operando XAS J. B. Leriche et al. J. Electrochem. Soc., 157, (5) A606-A610 2010 |

| Multicell support |
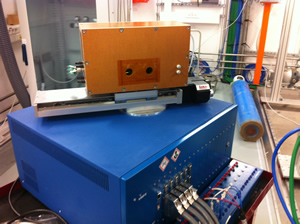
| Furnace RT to 90°C for 2 electrochemical cells for example |

| Thermostated support |
Notices
EXAFS :
gnuplothowto.pdf (114.69 KB - pdf)
GnuplotHOWTO.pdf
QuickExafs
quick-exafs_anglais.pdf (1.4 MB - pdf)
TBT_cryostats.pdf
tbt_cryostats.pdf (54.91 KB - pdf)
Information for the users, help for preparation and submission of proposal.
Help for project submission
How to prepare your Beamtime application ?
Project must be written in english and submissions must comply to the following framework :
Online submission of the web form to give general information
- Description of the scientific background and experimental part
- Figures or images in annex (format .jpeg .png)
- Description of experimental conditions with special safety measure
- Submission of the proposal
(If the application constitutes the project continuation, filing in a report of the previous experiment in the SunSet and mention of related publications are necessary)
Also,
- It is strongly advised to contact one of the beamline scientist, they will assist in the definition of the setups necessary for the desired experiment and in the assessment of the best conditions.
- The justification of the required beamtime is required.
Do not forget to mention your related publications in the sunset.
For more informations : The general User's guide
Préparation des échantillons
Gaz
The application for the use of specialty gases must be stipulated in your proposal in order that the security service can assess the associated risks. Specialty gases must be commanded 8 weeks before your experiment, also you have to prevent your local contact well in advance, for common gases (helium, argon, nitrogen, hydrogen, air and oxygen) only 2 weeks are sufficient.
Helium liquid
The application for the use of helium liquid must be stipulated in your proposal and recalled to your local contact in such a way to order sufficiently in advance the suitable volume.

