X-ray Absorption Spectroscopy to Study Redox and Structural Evolution in Na-ion Batteries
As sodium (Na) is more abundant and therefore less expensive than lithium, the Na-ion batteries are expected to be an alternative to the Li-ion batteries (LIBs) in energy storage systems. However, the energy density of Na-ion batteries is lower than that of LIBs. One way to increase energy density is to utilize the anionic redox in addition to the traditional cation (the transition metal) redox. Researchers from the Key Laboratory for Renewable Energy of Institute of Physics, Chinese Academy of Sciences (IoP, CAS) have studied the anionic redox strategy on Na-ion battery electrode materials, using absorption spectroscopy techniques (XANES-EXAFS) at the ODE and ROCK beamlines.
Cathode materials with high energy density, long cycle life, and low cost are of top priority for energy storage systems. Anionic redox is another important way to increase the energy density. However, there is still room for improvement in this technology, which currently leads to rapid and significant degradation of the electrode material and limits the number of charge/discharge cycles of the battery.
The cathodes of Na-ion batteries are generally layered transition metal (TM) oxides (NaxTMO2, x < 1), the TM often being manganese, which, like sodium, is abundant and harmless to the environment. But several reactions that occur in these cathode materials are damaging to battery operation: the Jahn-Teller geometric distortion from some of the octahedral Mn3+ ions-those with high spin at low potentials- which is one of the reasons for the severe structural degradation during the charge/discharge processes, and decrease in capacity of the Mn-based layered electrode; oxygen release triggered by oxygen redox.
Scientists from the Key Laboratory for Renewable Energy of Institute of Physics, Chinese Academy of Sciences (IoP, CAS) found that introduction of vacancies in the TM layer is effective in stabilizing the structure of the layered oxides at both the high and low potentials. On the one hand, the introduction of vacancies facilitates the oxygen redox and its reversibility without loss of oxygen. On the other hand, these TM vacancies increase the percentage of low spin Mn3+ ions in the discharged states, thus avoiding the distortion problem.
Thanks to a collaboration with the teams of the ODE and ROCK beamlines, the redox of Mn and the local structural changes within the electrode material Na2/3[Zn1/9Mn7/9□1/9]O2 have been characterized by X-ray absorption spectroscopy (XANES and EXAFS techniques). In this electrode material, an equal amount of Mn atoms is either substituted with more electronegative Zn atoms or removed to create vacancies.
The use of both ODE and ROCK beamlines presents the following advantages:
- XANES is a powerful technique to characterize the valence of TM in bulk material;
- EXAFS is very effective in providing structural information at the atomic scale to find out the impact of the oxygen redox on the coordination environment of TM ions, such as Mn-O and Mn-Mn bonding during the charge/discharge process;
In this study, the EXAFS results, together with the fitted bond length, significantly enhance the conclusion of the existence of low-spin Mn3+ in the materials at discharged state.
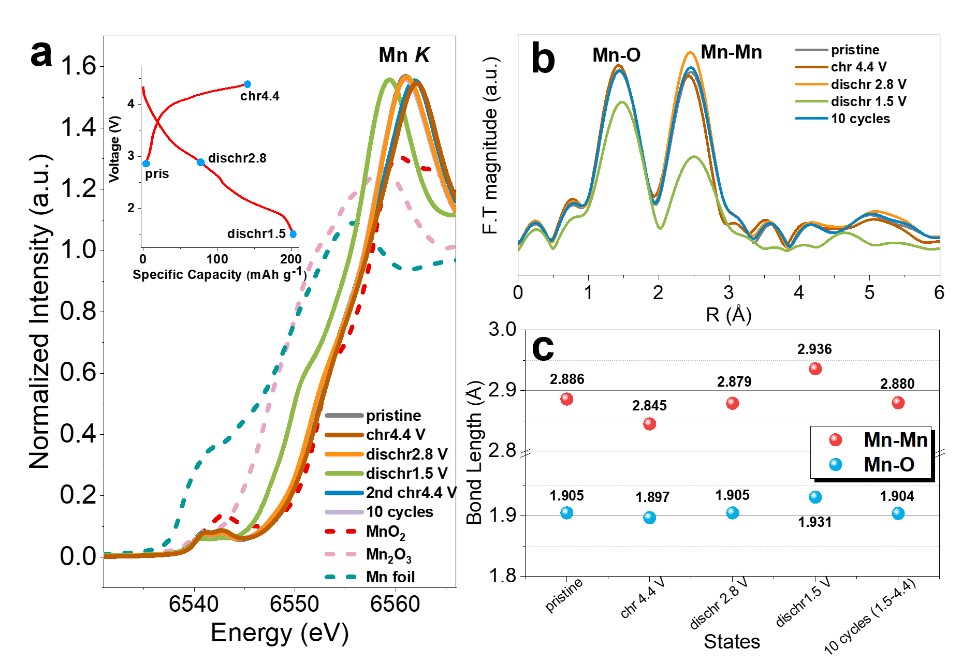
Figure 1a shows the normalized Mn K-edge XANES spectra at various states. The chemical state of manganese (Mn4+) remains unchanged during the initial charging, indicating that the oxygen donates the charge capacity. Study of the XANES spectra (Figure 1a) and of the Fourier transformed EXAFS spectra (Figure 1b) give access to the Mn redox process and the local structural changes. The reversible variation of the Mn-O and Mn-Mn bond lengths (Figure 1c) indicates the stable structure of the material during cycling.
This study inspires new ideas on designing cathode materials with high energy density and high structural stability and helps understand the role of the TM vacancies in enhancing the structural stability and in improving the thermodynamic and kinetic performances of cathode materials.
The normalized Mn K-edge XANES spectra at different states (a),
the inset is the voltage profiles of Na2/3[Zn1/9Mn7/9□1/9]O2). The Fourier transformed EXAFS spectra (k-weight = 3) (b)
and the fitted bond lengths from the Mn EXAFS spectra (c).