Withstanding pressure without losing color
High pressure is emerging as a powerful tool in structural biology, offering unprecedented access to intermediate folding states of proteins. At the Léon-Brillouin Laboratory (Saclay), in collaboration with the University of Belgrade (Serbia), scientists have studied the stability of R-phycocyanin (R-PC), a vibrant purple algal protein, by combining small-angle X-ray scattering (SAXS) under pressure at the SWING beamline with absorbance and fluorescence measurements.
Their analyses reveal major structural transformations and a partial preservation of the optical properties of R-PC, unlike what is observed after thermal denaturation. This study thus opens new perspectives for its use as a natural food colorant.
A new tool for structural biology
Initially used to explore extreme environments, high pressure (HP) is now becoming an innovative tool in structural biology. Unlike classical methods of thermal or chemical denaturation, it provides access to intermediate folding states of proteins without causing aggregation. As a key thermodynamic factor, HP promotes compact structures and, when applied moderately (up to 6,000 bars or 600 megapascals), highlights rare but essential conformations vital to protein activity. In parallel, HP, also known as "cold pasteurization", has been developed at an industrial scale, offering a more sustainable food preservation alternative by reducing energy costs, avoiding chemical additives, and preserving the integrity of active substances.
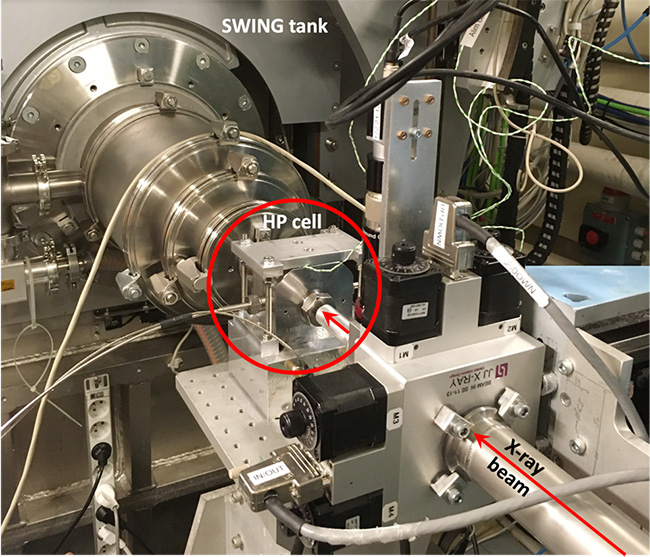
However, despite its immense potential, HP remains underutilized in structural biology. At the Léon-Brillouin Laboratory (LLB, UMR 12 CEA, CNRS, University of Paris-Saclay), researchers are exploring protein stability under pressure by combining small-angle X-ray or neutron scattering (SAXS/SANS) with optical spectroscopy (absorbance, fluorescence). In collaboration with the University of Belgrade (Serbia), the first results obtained from SAXS on biological macromolecules using the HP cell at the SWING beamline are presented here (Figure 1).
Unraveling the stability of R-phycocyanin under pressure
This study focuses on phycobiliproteins (PBPs), natural pigments found in algae, which are used as dietary supplements (spirulina) and natural colorants. Among them, R-phycocyanin (R-PC), extracted from edible red algae, stands out due to its oligomeric structure and fluorescent properties.
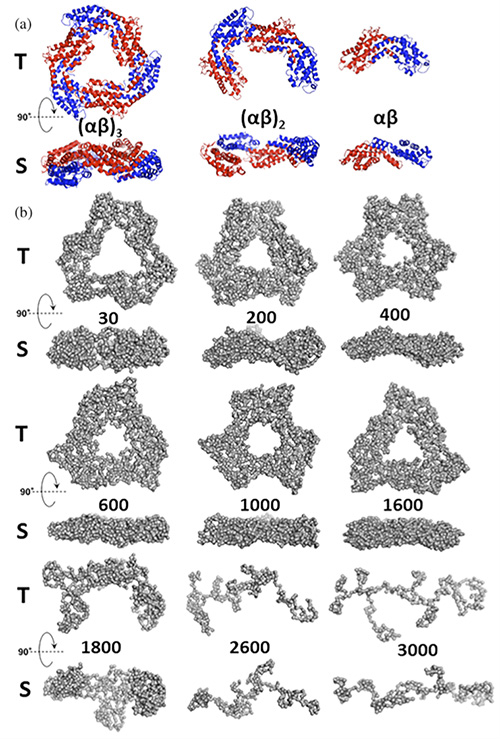
By combining HP-SAXS, visible absorption spectroscopy (HP-VIS), and fluorescence (HP-fluorescence), the effect of pressure on the stability of R-PC was analyzed. The HP-SAXS results reveal several major transformations: compression and flattening of the protein's trimeric structure, dissociation into monomers and dimers, and progressive elongation and unfolding of the monomers (Figure 2). These changes, confirmed by HP-VIS and HP-fluorescence, highlight the importance of these techniques for studying the behavior of PBPs under pressure.
Renaturation and potential applications
Upon depressurization (from 4,000 bars to atmospheric pressure), partial renaturation is observed, but without complete restoration of the native trimeric state. A mixture of monomers and dimers persists, accompanied by an increase in β-sheets at the expense of α-helices. However, HP better preserves the color of R-PC than heating at 60°C, thus maintaining its optical properties for use as a natural food colorant.
This study offers new insights into the unfolding and stability of PBPs under pressure, paving the way for innovative strategies for their preservation and use as natural colorants. Already industrially used for food preservation, HP could find new applications in the food industry. Future research will explore the effect of different food ligands, such as sugars, on the complete renaturation of R-PC. These results will also be compared to those obtained on C-phycocyanin (C-PC), which will be the subject of a forthcoming scientific publication.