Nitrogen cages to store hydrogen
Remarkable NHx compounds, obtained by compressing N2-H2 mixtures
Research groups from CEA, CNRS1 as well as the SOLEIL and ESRF synchrotrons, have studied compounds formed by compressing N2-H2 mixtures. One of these mixtures, in particular, with the formula (N2)6(H2)7, created a unique structure whereby nitrogen cages surround hydrogen molecules. By applying a very high pressure (higher than 50 GPa), followed by decompression, it was possible to generate both ammonia NH3 and hydrazine N2H4. This result demonstrates the ability to induce a chemical reaction under pressure without the need for a catalyst, using mixtures of simple molecules. This discovery could be of great interest in the synthesis of new high energy density matter (HEDM), or material capable of storing large amounts of hydrogen. This study has just been published in Nature Communications.
Although the importance of nitrogen for growing plants has been known for centuries, only natural sources of fertilizer were used at first (notably guano),including coveted and exhaustible deposits. To overcome this dependence on natural deposits, scientists have tried to find an alternative solution. By combining the effects of pressure with temperature and a catalyst, the German chemist, Haber, managed to convert atmospheric nitrogen into ammonia. The Haber-Bosch industrial scale application of this process led to the mass production of fertilizers from 1913 onwards.
Many studies have since been published on the properties of hydrogen and nitrogen at very high static pressures. A polymeric form of nitrogen was thus synthesized above 100 GPa (i.e. nearly a million times atmospheric pressure), making this the material with the highest density of stored energy. Meanwhile, metallic hydrogen has been the subject of intense research because it could have unique properties, such as the ability to conduct electricity without loss (superconductivity) at room temperature. In order to combine these two properties (high energy density and high temperature superconductivity), scientists are now looking to combine nitrogen and hydrogen with other elements under high pressure. Yet, until now, the properties of N2 and H2 mixtures under high pressure have remained unexplored.
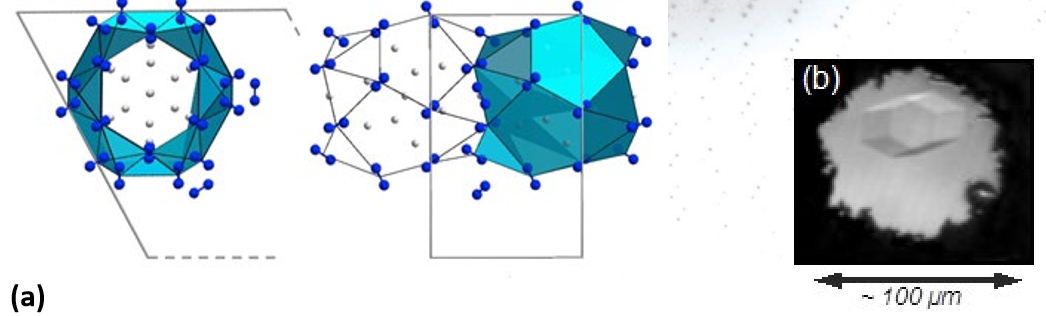
In this study, the researchers looked at different N2-H2 concentrations under pressure. These mixtures solidified at between 3 and 7 GPa, with the formation of two compounds, (N2)6(H2)7 and N2(H2)2. The compound (N2)6(H2)7 had a particularly interesting and completely original structure; wide molecular nitrogen cages surrounding 14 hydrogen molecules (see Figure 1). This is the first example of such a clathrate-type structure in which very low van der Waals forces form the basic structure of the compound and not strong chemical bonds between the atoms.
Increasing the pressure (50 GPa) on this cage structure promoted a chemical reaction between the nitrogen and hydrogen molecules and a new compound was then formed, consisting of nitrogen and ionized ammonia. With a drop in pressure, a nitrogen and hydrazine (N2H4) mixture was recovered. A pressure cycle has enabled the synthesis of an energetic material, hydrazine, used in particular as rocket fuel, starting only with nitrogen and hydrogen, and therefore pollutant-free (which is impossible at ambient pressure). These results thus open new avenues to the «clean» synthesis of energetic materials.
1 Institut de minéralogie, de physique des matériaux et de cosmochimie (IMPMC, CNRS/UPMC/IRD/MNHN)