New high-pressure/low-temperature setup
Setups build to carry out high-pressure spectroscopy experiments –infrared (IR), TeraHertz (THz) or Raman– have been presented over the past years, some of them combining high pressure and low temperature and using synchrotron radiation. Scientists from the AILES beamline in collaboration with the GREMAN have developed a setup for its versatility and to take advantage of SOLEIL high stability and brilliance in the infrared and THz ranges.
In condensed matter, exploring the phase diagram as functions of thermodynamic parameters is a suited way to modify properties of samples in order to understand the microscopic mechanism behind these properties. The synchrotron infrared (IR) light, several times more brilliant than conventional IR sources, is ideal to be coupled with setups requiring high photon flux, for instance to high pressure setups based on a diamond anvil cells (DAC). Moreover, diamonds being transparent in the IR and THz ranges are well suited for DAC based transmission or reflectivity experiments.
The design and development of the new high pressure low temperature (HPLT) set-up available at the AILESbeamline has been realized so that it could meet the following requirements:
- The pressure applied to the sample can reach at least 20 GPa;
- The set-up has to be compatible with a wide spectral range (all IR regions down to 25 cm-1);
- The temperature of the sample can reach 20 K;
- The set-up allows for in situ change and reading of the pressure within the cell;
- The optical set-up allows for measurement of IR transmission or IR reflectivity.
By combining the synchrotron radiation light available on the AILES beamline with the new HPLT setup, one can minimize the relative noise in the transmission configuration down to 25cm−1 and until 60cm−1 in the reflectivity configuration with a high signal to noise ratio.
FIR absorbance of “bulk” water, under high pressure and low temperature
The phase diagram of ice is complex with 18 crystalline phases and three amorphous phases. All these crystalline phases involve water molecules bound to four other molecules by H-bonds and in most cases the two hydrogen atoms are equivalent. Lowering the temperature leads to proton ordering whereas increasing the pressure will reduce the distances between second shell neighbors. Infrared spectroscopy is a technique of choice for studying the establishment and the conformation of the hydrogen bond. In particular, in the far infrared domain, one directly probes the intermolecular modes namely the librational modes (frustrated rotation of H-bonded water molecules) and the connectivity band (stretching vibration between two H-bonded water molecules).
The DAC was filled with liquid water without any medium of pressure. Only ruby balls were added in order to evaluate the pressure inside de DAC. Measurements were performed, in transmission in the far-infrared domain, between 25 cm-1 and 550 cm-1, by means of synchrotron beam, combined with the interferometer equipped with a 4.2 K bolometer and a 6 µm Mylar beamsplitter. For each spectrum, 50 scans at 1 cm-1 resolution were co-added.
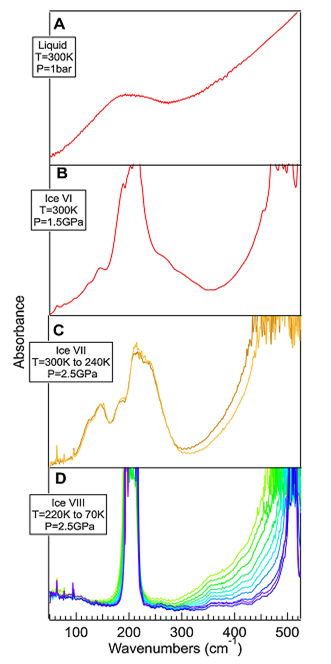
Figure 1 presents absorbance spectra of liquid water (Figure 1A) and ice (Figure 1B–D). In the liquid phase (Figure 1A) the connectivity band is centered around 180 cm-1 and is superimposed on the low energy side of the librational band. With the transition in ice, the former becomes narrower and shifts toward higher frequency while the librational mode narrows down. As the connectivity band is a direct probe of the H-bonds, the structural changes and, more so the proton ordering, cause strong modification to the spectral profile with each type of ice providing a different spectral signature.
The new set-up has allowed investigating three phases of solid water. Indeed, as illustrated in Figures 1B–D, the FIR absorbance spectra of each type of ice are strongly modified compared to liquid water. In particular the transition between liquid and ice VI gives rise to narrower connectivity bands representative of the ordering of the molecules in a solid network. The Ice VII presents a complex FIR signature which is related to the two interposed network of ice. Finally, ice VIII shows further modifications of the far infrared spectra with the appearance of two strong and narrow bands (although saturated), at 200 cm-1 and 510 cm-1, which can clearly be related to the proton ordering.
In conclusion the HPLT device now available at the AILES beamline permits studies by IR spectroscopy of condensed matter in a wide range of thermodynamics conditions. The high stability of the SOLEIL synchrotron source combined with the high positional stability of the device allows very accurate optical measurements and an optimal extension to the THz range.