Nanocrystals and droplets: a miniature system to study the impact of proteins on biomass components
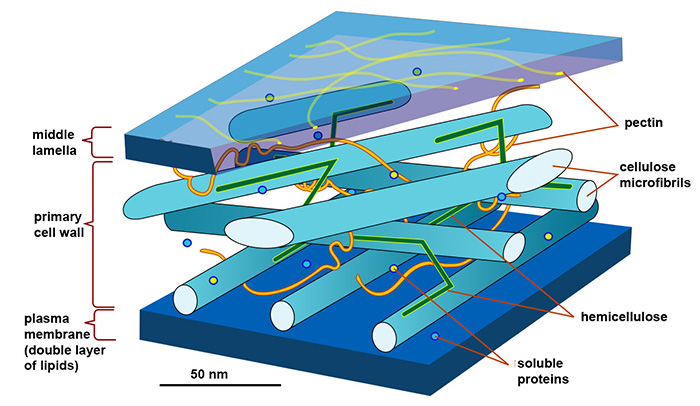
The production of agrifoods, biofuels or biobased materials can involve the transformation of biomass. At the molecular level, cellulose polymers, a major polysaccharide component of plant matter, are transformed via reactions involving proteins such as enzymes.
To understand these complex phenomena, scientists from INRAE, ESPCI and SOLEIL's DISCO beamline have designed an experimental platform for visualizing proteins in a dense, organized cellulose system similar to that found in plant cell walls. The platform is a microliter drop containing cellulose nanoparticles.
Biomass can provide answers to a number of current technological and societal challenges, including the development of renewable biofuels, "platform molecules" (building blocks for the chemical synthesis of other, more complex molecules) or new food resources. Advances in this field are based on an understanding of polysaccharide-protein interactions. However, studies are usually carried out in dilute media, which do not reflect the composition of plant matter. Consequently, new strategies are needed to study proteins under constrained conditions, i.e. in highly concentrated and organized media, like plant cell walls. The aim of this study is to create an experimental platform for simulating the dense organization of cellulose in plant cell walls, in order to monitor the localization of proteins as they exist in biomass, for food applications or enzymatic degradation of plant walls.
Cellulose-protein interactions were studied in a suspension of cellulose nanocrystals (CNC), a model form of cellulose. Among other properties, these nanocrystal suspensions are known to self-organize into a liquid crystal phase called the cholesteric phase, which approximates the organization of cellulose fibrils in the plant cell wall (figure 1).
This liquid crystal structure was obtained in droplets of these organized suspensions, which were then trapped in an ad hoc microfluidic device. They were used to study the behavior of two model proteins: bovine serum albumin (BSA), a model protein for food systems, known not to react strongly with cellulose, and the endoglucanase enzyme GH7 which, unlike BSA, binds to the molecules it degrades -polysaccharides, like cellulose- via its polysaccharide-binding module.
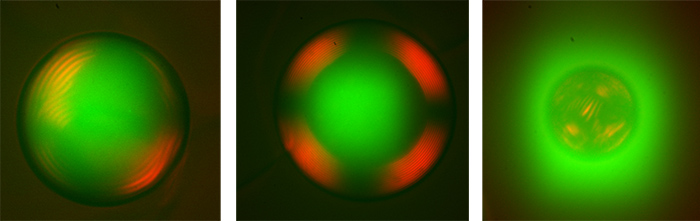
CNC droplets organized in cholesteric phase and protein distribution were studied using deep UV synchrotron radiation (DUV) on the DISCO beamline. The principle is to take advantage of the autofluorescence of proteins when they are illuminated by DUV, to localize them in UV microscopy without disturbing their structure and activity.
When a molecule or particle of interest is studied, it usually has to be tagged with fluorescent chemical groups beforehand, so that its localization can be followed by microscopy. However, this labelling can interfere with the activity of sensitive proteins such as enzymes. On the DISCO beamline, thanks to the combined use of the microfluidic device and DUV microscopy, whole droplets of CNC could be observed, within which BSA and GH7 were localized in real time thanks to their autofluorescence. In addition, cross-polarization microscopy enabled us to characterize the state of organization of these proteins in the concentrated cholesteric envelopes that make up the droplets.
It was thus observed that the proteins were homogeneously distributed throughout the droplet volumes, unaffected by the isotropic or cholesteric state of the cellulose; however, the organization of the CNC reacted very differently depending on the protein.
The usually inert BSA strongly disrupted the overall droplet organization by preventing the formation of a large cholesteric domain. DUV microscopy also revealed that BSA was excluded from continuous CNC droplets to diffuse into surfactant micelles present around the droplets, constituting a "micellar engine", which is not the case for GH7. While no objective quantification was possible, these results highlight that polar, low-energy interactions occur between cellulose and BSA, while GH7, which naturally interacts with cellulose via binding modules, retained its 3D structure unchanged.
These results demonstrate how proteins can behave in densely organized cellulose environments, and conversely the impact of such an environment on protein behavior. This work can be used for a variety of biological applications, such as the study of enzymatic action on biomass during bioprocessing, the study of protein-polysaccharide interactions in innovative biobased materials, or the understanding of digestion in various food processes.
In further work, the proportion of CNC-ordered domains will be modulated and the system complexified. In particular, other biopolymers such as hemicelluloses will be added to more closely reproduce the composition and structure of the plant cell wall, and to assess their influence on the protein behavior of the enzymes.