A molecular catalyst able to form carbon-carbon bonds from CO2
Carbon dioxide (CO2) is a natural greenhouse gas, whose overproduction by humans (notably through the combustion of fossil fuels) contributes to global warming. But this industrial waste is also a potential economic opportunity: CO2 can be used to produce essential chemical products without the need for oil, or to store energy from renewable and intermittent sources in the form of fuels.
How can this be done? Through electrochemical reactions requiring the use of catalysts.
In this study, a molecular catalyst composed of abundant elements (C, H, N, Fe) was used to electrocatalytically reduce CO2 to light hydrocarbons. The results show that this catalyst can form carbon-carbon bonds, opening the way to complex chemical processes.
Electrocatalytic reduction of CO2 is a promising technology for converting this abundant industrial waste into commodity chemicals or fuels. Since CO2 is an inert molecule, catalysts are vital to make the process more efficient and thus, more economically viable. Most catalysts only yield single-carbon (C1) products, while copper-based catalysts are outliers with the ability to produce more valuable multi-carbon (C2+) compounds. However, after several decades of research, product selectivity is still a problem for copper catalysts, and the detailed mechanism of CO2 reduction on copper is still debatable.
On the other hand, macrocyclic molecular catalysts are known for producing C1 compounds with excellent selectivity, while the nature of their active sites allows the study of detailed reaction mechanisms. In this study, scientists of the LUCIA beamline explored the ability of one of these catalysts to produce compounds with two or more C-C bonds (C2+) to propose new strategies for the optimization of the CO2 reduction reaction.
Unexpected C-C bond formation
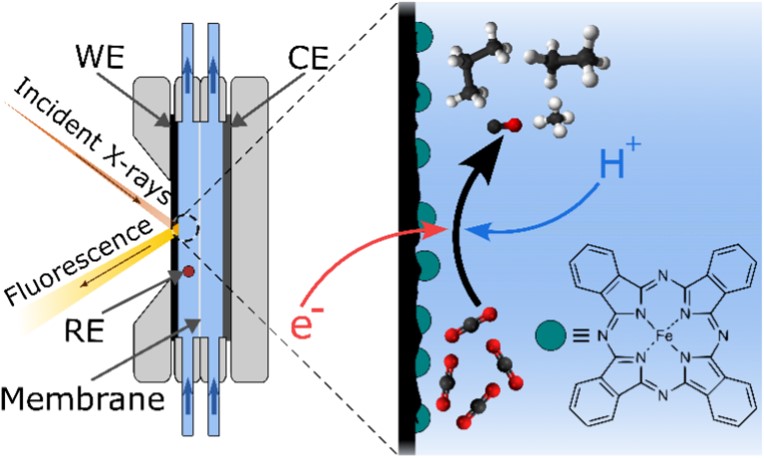
In order to reduce CO2 electrocatalytically, Iron phthalocyanine (FePc) was selected. It is a commercially available catalyst composed of abundant elements only: carbon, nitrogen, hydrogen, and iron. This molecule was deposited as a thin film on a carbon electrode and inserted into a flow-through electrolysis cell (Figure 1), which also allows for performing X-ray absorption spectroscopy experiments during catalysis. Under optimized conditions, FePc yields 80% carbon monoxide (CO) and 20% hydrogen (H2). The initial objective was to obtain a maximum of CO. But, a handling error led to the deposition of more catalyst on the electrode and the observation of unexpected products.
Indeed, the gas chromatography analysis of the gaseous products shows the presence of light saturated and unsaturated hydrocarbons, such as ethylene, ethane, propylene, propane, butene, and butane. These compounds have two to four carbon atoms, which implies the formation of carbon-carbon (C-C) bonds during the catalytic reaction.
Origin of the carbon atoms and catalyst stability
The hydrocarbons obtained during the reaction are in minimal quantities (less than 1%). Several control experiments were carried out to ensure the origin of the carbon atoms and the stability of the catalyst. Experiments performed in the absence of CO2 (but in the presence of the catalyst) or in the absence of the catalyst (but in the presence of CO2) did not produce any hydrocarbons, indicating that CO2 and the catalyst are both required to produce C2+ compounds. Gas chromatography coupled with mass spectrometry measurements were performed in the presence of carbon-13 labeled CO2, showing the carbon atoms in the detected hydrocarbons did come from CO2.
Moreover, X-ray absorption spectroscopy experiments performed during the catalytic reaction on the LUCIA beamline of SOLEIL (see figure 2) demonstrate the chemical stability of the catalyst during and after the reaction.
Conclusion
The study of minority products obtained during the electrocatalytic reduction of CO2 by iron phthalocyanine have shown for the first time that this catalyst can form light hydrocarbons from C1 to C4 containing up to three C – C bonds. Infrared and X-ray absorption spectroscopic measurements have shown that the catalyst retains its molecular structure intact during and after the catalytic reaction. These observations open the door to the improvement of this family of catalysts: their chemical structure can be modified to optimize the selectivity towards these light hydrocarbons.