Mixed valence compounds: TEMPO beamline reveals the divalent character of hydrated Europium salt in Ultra High Vacuum
Applications using Europium ions and especially Eu3+ ions know renewed development in photonic applications such as organic light emitting diodes (OLEDs), lasers, optical communications, and as luminescent tags in biological systems. The Eu2+ ions don’t have the same interesting optical and electronic properties as Eu3+. Scientists from LPCML, ICMR and SPCSI used photoemission spectroscopy on TEMPO to show that EuCl3 salt contains the Eu3+ expected ions, but also a notable proportion of Eu2+ ions, as a stable form under vacuum.
Lanthanides ions present good photonic qualities such as spectral purity. Europium ion is one of them and has been used for a long time as a dopant in organic phosphors (used in cathodic-ray tubes for example). Recently, lanthanide ions have shown new potentialities for qubit applications. Nevertheless, as a restriction to applications, their high sensitivity to ionic or molecular surrounding affects their optical properties.
The sharp atomic-line-like red luminescence of the Eu3+ ions is at the origin of the interest for applications. The emitted light mainly originates from the electronic transition inside the partially filled 4f electron shell. In contrast, Eu2+ exhibits a broad emission band in the UV exhibiting poor quantum efficiency.
Eu2+ or Eu3+?
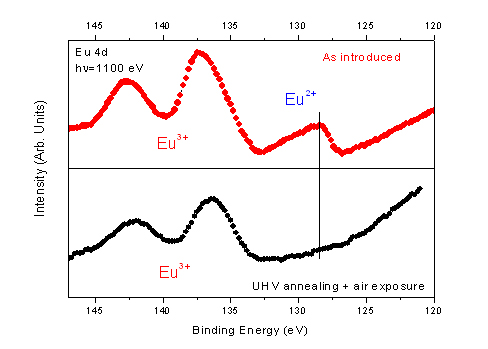
Although hydrated EuCl3 salt are well characterized and stable species, chemical dosing of commercial salt has revealed an unexpected chemical composition of one europium ion for 2.85 chlorine counter ion. However, optical luminescence of the salt measured in air exhibits a pure Eu3+ spectrum. In order to assign the electronic properties of the hydrated europium salt we have investigated Eu 4d core level electronic configuration by means of photoemission. The salt was solubilized in dry ethanol and drop-casted on a gold substrate. Surprisingly, the photoemission spectrum of europium 4d core level presents a singular asymmetric shape component attributed to Eu2+ species, thus revealing a significant amount of divalent europium, top of Fig. 1. Europium ion is very sensitive to sample preparation. Indeed, UHV annealing of the sample at a temperature larger than 350°C followed by air exposure lead to pure Eu3+ oxidized species, bottom of Fig. 1
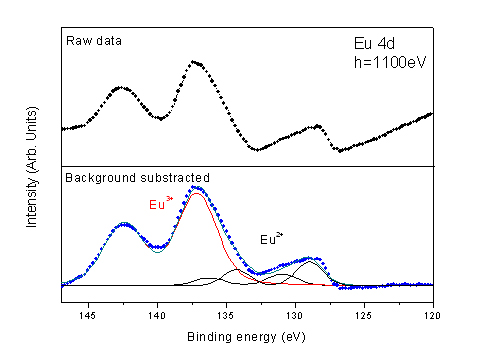
By Eu 4d core level spectrum curve fitting, the Eu2+ to Eu3+ ratio was determined quantitatively (Fig. 2). Comparing the Eu2+ and Eu3+ contributions, a proportion of 15-20% of Eu2+ is found in the hydrated Europium salt. This result is in good agreement with the chemical dosing revealing a presence of 2.85 chlorine ions for one europium ion.
This work has shown that initial hydrated europium salt presents stable phase under vacuum forming a mixed valence hydrated EuCl2.85 compound.
The understanding of electronic structure and chemical stability of europium salt in air and UHV is a key factor to improve such material processing and a prerequisite to the study of more complex systems such as organometallics. Indeed, isolated europium ions present very low absorption cross section in UV. To overcome this limitation europium ion has to be associated with an organic ligand playing part of antenna and increasing their optical absorption efficiency.